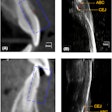
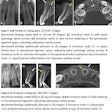
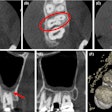
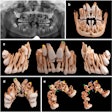
3D Diagnostic Imaging, parent company of CarieScan, reported losses of 2.4 million pounds ($3.7 million) on revenues of 715,000 pounds ($1.1 million) for fiscal year 2011 (end-June 30).
The company reported losses of 1.2 million pounds ($1.9 million) on revenues of 2,800 pounds ($4,000) for the previous 12-month period.
3D Diagnostic Imaging attributed the increases in losses to a manufacturing defect in the CarieScan Pro device and costs related to the opening of a U.S. sales office. The company has now closed its U.S. office, eliminated its U.S. sales force, and turned over North American sales efforts to CoreStrength.
"The year ended 30 June, 2011, was one of mixed fortunes for 3D," said James Noble, chairman of 3D Diagnostic Imaging, in a press release. "Whilst sales of the CarieScan Pro in the USA were negatively impacted following the identification of a manufacturing defect, the management team responded well, and I am pleased to report that this defect was quickly rectified and that the product has been relaunched in the U.S. with an enhanced sales support operation."
In the meantime, the company has been focusing on territories outside the U.S. and has now signed a number of distribution agreements in Europe, China, and India.
The company has also taken a number of other measures to strengthen the business, including securing 1.41 million pounds ($2.2 million) of additional equity funding.