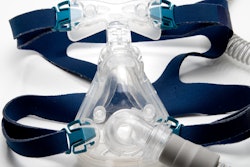
Philips will halt sales of its bilevel positive airway pressure (BiPAP) and continuous positive airway pressure (CPAP) machines under a tentative agreement it reached with the U.S. Food and Drug Administration (FDA) and the U.S. Department of Justice (DOJ). The deal, which primarily affects Philips Respironics devices, has not been finalized and will need to be approved by a U.S. judge, according to a Philips news release dated January 29.
Philips will service previously sold sleep and respiratory care devices, but it will not sell new CPAP or BiPAP devices or other respiratory care devices in the U.S. “until the relevant requirements of the consent decree are met,” according to the release.
Furthermore, Philips has set aside $393 million to pay for remediation activities and disgorgement payments for Respironics devices in the U.S.
The latest development ends years of manufacturing issues that have dogged the company.
In December 2023, the FDA issued a safety communication that Philips’ Respironics machines should be monitored for signs of overheating following reports of fire and burns. The agency issued the advisory after noting an increase in medical device reports associated with DreamStation 2 CPAP machines.
Between August 1, 2023, and November 15, 2023, the agency reported a sharp increase of more than 270 reports of thermal issues associated with the machines. In November, Philips said in a news release that based on an analysis, the machines could continue to be used provided that consumers used them according to the safety instructions.
In 2021, Philips voluntarily recalled some of its Respironics sleep and respiratory care products due to the potential for the polyester-based polyurethane foam used to manufacture the devices breaking down over time, resulting in consumers either swallowing or breathing in the foam or chemicals.
In September 2023, Royal Philips and its U.S. subsidiaries, including Philips Respironics, reached a proposed settlement totaling more than $618 million. That settlement would offer cash awards to eligible U.S. participants depending on the type of device and extended warranties on replacement devices. Philips said there would be additional compensation for consumers who returned the units to Philips Respironics. Final approval has not yet been granted.
More details about the consent decree are forthcoming once it has been finalized and submitted to the relevant U.S. court, according to Philips.