University of Louisville researchers are one step closer to helping millions of people whose salivary glands no longer work because of disease or damage from disease treatment.
Douglas Darling, PhD, a professor in the department of oral health and rehabilitation at the university's School of Dentistry, and his team have identified a protein-sorting mechanism used by the salivary gland thatcould form the basis for advanced therapies for patients whose salivary glands are damaged or no longer function due to radiation therapy, prescription drugs, or Sjögren's syndrome (Journal of Dental Research, May 31, 2011).
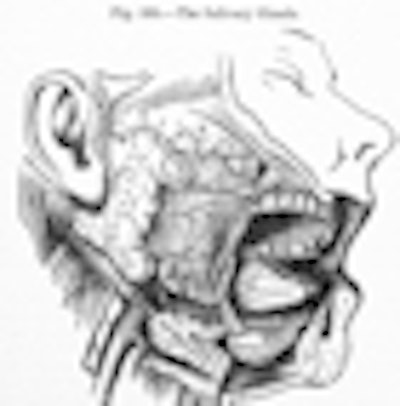
The largest of the salivary glands -- the parotid -- secretes important proteins into the saliva. Because it has multiple secretion pathways, it must sort proteins destined for saliva into the correct pathway for secretion.
This can be tricky as there are seven possible pathways, according to Darling and his colleagues. One pathway takes proteins to the salivary duct, while other pathways carry different proteins to the "back" side of the cell to be secreted into the blood or to form a supportive matrix for the cells. Transport along these pathways occurs by sorting the proteins into vesicles that carry their "cargo" to the correct destination.
Conventional thought was that cargo proteins are moved into the forming vesicles by attaching to sorting receptor proteins. Darling and his team have discovered a completely new approach, suggesting the reason no salivary sorting receptor protein has been found is that it may not exist.
In this new model, the salivary cargo protein, parotid secretory protein (PSP), selectively and directly binds to a rare lipid, PtdIns(3,4)P2. This lipid is present only in certain cell membranes and only on one side of the membrane.
But Darling and his team found that the lipid can flip to the inner part of the vesicle membrane, giving PSP the opportunity to bind it.
"These data imply that phosphatidylinositol-phosphate lipids like PtdIns(3,4)P2 may have multiple functions on the inner surface of organelles," Darling stated in a university press release. "This is contrary to the current belief that their functions are always limited to one surface of the cell membrane."
The next step is for Darling and his team to identify the molecular components used for flipping PtdIns(3,4)P2, and develop approaches to test ways to manipulate this potential protein sorting mechanism.