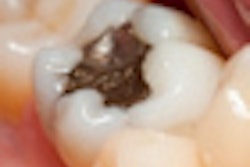
Amalgam opponents plan to formally petition the FDA this week for reconsideration of its July 28 guidelines for the material, part of a four-pronged approach by which they hope to eliminate mercury from dentistry.
They are also demanding an investigation of FDA Commissioner Margaret Hamburg, M.D., for an alleged conflict of interest. They're planning legal action against the agency for allegedly violating a settlement in which it issued warnings about amalgam. And they're lobbying members of Congress, said Charles Brown, national counsel for Consumers for Dental Choice.
"We're going to fight and we're going to win," said Brown, author of a lawsuit that forced the FDA to post warnings about amalgam on its Web site last year. "The FDA is not going to get away with this."
Petition
“We're going to fight and we're going to win.”
— Charles Brown, national counsel
for Consumers for Dental Choice
Brown said a coalition of groups opposed to mercury in dentistry would file a petition for reconsideration of the ruling by a September 3 deadline. The petition will argue that the FDA did not adequately consider evidence that mercury in amalgam poses a health risk.
The July 28 guidelines require makers of amalgam filling materials to disclose potential risks from mercury, but only contraindicate it for people with specific allergies to the material. In announcing its decision, the FDA said it had considered more than 200 peer-reviewed studies on amalgam, and had also taken comments from the public. Brown argues that the evidence shows amalgam is unsafe and insisted that most of the public comments were on his side.
Consumers for Dental Choice, however, will not be named in the petition because that would undermine its legal status as a party to its settlement with the FDA, Brown said. Instead, other groups will put their names on the petition while Consumers for Dental Choice seeks to remedy what it sees as a breach of the settlement.
What was the breach? The FDA violated its agreement to post on its Web site a warning that amalgam might pose a risk of neurological damage to "developing children and fetuses," Brown said. The agency now says that the scientific evidence suggests that even these groups may safely have amalgam restorations, but it has maintained the warning on a page of its site.
Brown is also incensed that the FDA is allowing materials makers to use the term "silver" in describing amalgam, since the primary ingredient is mercury.
Conflict alleged
The FDA's ruling "was a big surprise," Brown said. In seeking to explain it, he points the finger at Dr. Hamburg's relationship with Henry Schein. He has written to the Office of the Inspector General of the U.S. Department of Health and Human Services, requesting a formal investigation of those connections, and of "inappropriate industry influence" on Daniel Schultz, M.D., former director of the FDA's Center for Devices and Radiological Health.
Dr. Hamburg served on the Schein board of directors and owned both stock and stock options in the company. Under federal ethics guidelines, presidential appointees are not allowed to receive any outcome or "hold financial interests that conflict with the conscientious performance of duty."
A spokesman for the inspector general said he would not comment on the possibility of an investigation. But Dr. Hamburg "was not involved in the decision-making for the rule on dental amalgams," according to an FDA statement.
Also between her May 18 Senate confirmation and taking office on May 26, Dr. Hamburg "resigned her position as Board Director at Schein, sold all of her Schein stock, exercised all the Schein options she owned that had monetary value, and immediately, that same day, sold all the resultant stock," the FDA noted in the statement. "At that time, Dr. Hamburg also forfeited unvested restricted Schein stock that was worth $262,000."
The FDA goes on to say that she continued to own some options that had no value and that she couldn't sell them because they were "nontransferable." When some of these options gained value on July 16, Dr. Hamburg sold them for $2,593.73, and requested that Schein cancel her remaining options, which the company did on July 27, according to the FDA.
Brown argues that Dr. Hamburg should have publicly announced her recusal from the amalgam rule-making earlier in the process. He speculates that she actually did participate, if not directly, at least by allowing Dr. Schultz to oversee the process. Critics have charged Dr. Schultz with bowing to industry pressure to approve other devices. He resigned from the agency August 11.
Brown charges that Schein benefited from the amalgam ruling because it sells amalgam. He noted that the company's share price increased $2 per share the week the ruling was announced, and that Schein CEO Stanley Bergman thanked Dr. Hamburg for her serving on the board. (The company's stock price, which had been increasing since July 9, dipped by $0.24 the day after the FDA's ruling was announced.)
For its part, Schein denies that it influenced the FDA through Dr. Hamburg. "We believe the claims filed concerning Dr. Margaret Hamburg and Henry Schein, Inc. are without merit," the company said in a statement. "Dr. Hamburg resigned from the Henry Schein Board of Directors on May 19, 2009, upon her confirmation as Commissioner of the Food & Drug Administration. While Dr. Hamburg was a member of Henry Schein's Board, her role was limited to the general oversight of the Company. For background purposes, Henry Schein's sales of amalgam products are less than one half of one percent of the company's overall sales."
Schein sells composite resins as well as amalgams. But Brown argues that the company got a business advantage from the FDA ruling because amalgam materials are less expensive. In theory, at least, some patients might buy amalgam restorations who couldn't afford composite resins.
Lobbying
What if the formal complaints and the legal maneuvers fail? The amalgam foes' last ace in the hole is the sympathy of at least two members of Congress, Reps. Diane Watson (D-CA) and Dan Burton (R-IN). In May, the two circulated a petition among their congressional colleagues asking the FDA to require parents to sign a consent form before dentists can place amalgam restorations in children younger than age 18. Adults would have received a warning letter "to ensure they are fully aware of the potential harmful effects of mercury amalgam."
But an effective push from Congress isn't a sure bet, either. Two dentists in Congress, Reps. Mike Simpson (R-ID) and John Linder (R-GA), opposed the letter.
The only certainty is that the debate will continue.
Copyright © 2009 DrBicuspid.com