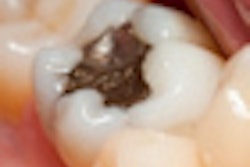
Amalgam opponents turned out in force September 22 at a U.S. Food and Drug Administration (FDA) town hall meeting in South San Francisco, CA.
It was the third in a series of such meetings the FDA has held this year to solicit feedback from citizens, entrepreneurs, and health professionals about dental amalgam, medical device regulation, and related topics.
The meeting was moderated by Jeffrey Shuren, MD, JD, director of the FDA's Center for Devices and Radiological Health (CDRH), who fielded a number of questions from concerned groups and individuals.
While much of the meeting focused on the 510(k) regulatory approval process for medical devices, the most impassioned pleas for change came from those who spoke about amalgam.
David Kennedy, DDS, a practitioner who advocates for mercury-free dentistry, spoke briefly before introducing Stacy Case, a television news anchor who specializes in consumer and investigative reports. Case, who has multiple sclerosis (MS), attributes her illness to her amalgam fillings.
"Nearly four years ago, I allowed a dentist to talk me into replacing four 30-year-old silver mercury fillings [with new amalgam fillings] because he said they were cracked," she said. "Your agency had done nothing to warn me, an educated professional, that this was a health hazard."
Case got the fillings because they were covered by insurance, she said. Several days after the new fillings were placed, a metallic taste developed in her mouth.
"Within a few weeks, I developed deep, painful headaches, and within months a myriad of neurological symptoms," she said. "The MS diagnosis came later. My immediate concern for my children, 2 and 3, was, 'Will I be caring for them or will they be caring for me?' " she said, choking up.
She claims there was a dramatic turnaround in her condition once there was an absence of amalgam in her mouth. "I became spontaneously well after having the fillings removed using the International Academy of Oral Medicine and Toxicology protocol, and undergoing IV chelation therapy," she explained. "I have been in remission for 3 years."
Another speaker, Anita Vazques Tibau, the California grassroots director for Consumers for Dental Choice, admonished the FDA for its lack of information about mercury in Spanish on the agency's website.
"Many Spanish speakers told me that their dentists have never ever used the word 'mercurio' in a discussion," Vazques Tibau said. "Instead, it is deceptively called 'la amalgam la plata -- 'silver fillings.' I'd like to see you tell everyone that mercury is toxic, and please post it in Spanish as well."
She angrily continued to ask why mercury was still being used in the U.S. when it has already been replaced in other nations.
"Dr. Shurer, you told European regulators that 'We don't use our people as guinea pigs,' but the FDA admits that mercury can cause neurological harm in children," Vazques Tibau said.
Mercury-free, atraumatic restorative treatments are already being used in Latin America, and the Pan American Health Organization has deemed them safe, less expensive, and able to be placed outside of clinics, Vazques-Tibua added.
Another speaker referenced one of the previous town hall events, where another FDA representative said the agency promised to address the issue of amalgam safety once and for all.
"Anthony Watson of the FDA concluded that meeting by saying, 'We're going to go back and really hit this, and hopefully we'll come out with something that we can all be proud of,' " the speaker noted. "My question is, when will the FDA come out with something we can all be proud of?"
In July 2009, the FDA issued a final rule that reclassified dental amalgam and its component parts as class II devices and designated special controls for the materials.
But the ruling caused an outcry from some consumer and patient groups that are convinced of the dangers of mercury and that are seeking a higher level of regulation.
"When I came to CDRH in late 2009, we had received concerns about prior assessments of the evidence in dental amalgam," Dr. Shurer responded. "One of the things I did was reconvene the advisory panel in December of 2010. And what I can tell you is we intend to come out with an announcement by the end of the year."