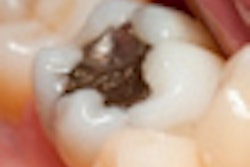
Labeling for dental amalgam should more clearly inform dental practitioners and their patients of the potential risks posed by the mercury it contains, an expert panel advised the U.S. Food and Drug Administration (FDA) at a two-day meeting in Gaithersburg, MD, this week.
And the FDA should do its own risk assessment and meta-analysis of scientific literature to gain an authoritative grasp of what is knowable about the dangers that mercury restorations may pose, particularly for sensitive subgroups such as pregnant women, their fetuses, young children, and medically vulnerable patients.
The FDA is expected to consider the panel's advice, delivered at the conclusion of the meeting, and then determine whether to make changes in how it regulates dental amalgam.
"We are going to go back and really hit this, and hopefully we will come back with something everybody can be proud of," FDA Dental Devices Director Anthony Watson, MBA, told the panel.
In July 2009, the FDA issued a final rule that reclassified dental amalgam and its component parts as class II devices and designated special controls for the materials.
But the ruling caused an outcry from some consumer and patient groups that are convinced of the dangers of mercury and that are seeking a higher level of regulation. They said the FDA underestimated the level of mercury exposure from dental amalgam and failed to fully consider differences among subpopulations that could affect the absorbed dose. They also contended that the reference exposure level (REL) of 0.3 µg/m3, set by the FDA to assess risk for mercury exposure, was not sufficiently protective.
Pages of expert reports
During the two-day meeting this week, the panel explored a range of mercury exposure scenarios for dental amalgam but did not collectively embrace any of them.
“Hopefully we will come back with something everybody can be proud of.”
— Anthony Watson, MBA, FDA dental
devices director
"The FDA has the best risk assessment experts in the world," said panelist Michael Dourson, PhD, an Ohio toxicologist. "Look at the new data and develop your own REL. By all means, use all the new tools we have."
At the request of the FDA, the panel weighed pages of expert reports and listened to testimony, much of it from dozens of speakers convinced they had been harmed by their amalgam fillings.
"My dentist poisoned my brain," testified Virginia housewife Marie Flowers, describing the onset of debilitating illness she blamed on dental mercury.
Others suggested the FDA itself has attempted to hide the dangers of amalgam.
"The FDA is under the influence of sinister forces that are trying to undermine health," said New Jersey dentist Steve Markus, DMD.
Yet others attested to the safety of the material, which has been used by dentists for more than 150 years.
University of Washington researcher Michael D. Martin, DMD, MPH, PhD, who spoke about the Casa Pia Study of the Health Effects of Dental Amalgams in Children (Journal of Toxicology and Environmental Health, Part A, December 2001, Vol. 64:7, pp. 521-530), said that he and other researchers found no statistically significant differences in memory, attention, visual motor function, or nerve conduction velocities over the seven years they compared the health of a group of Portuguese schoolchildren with amalgam fillings with a group that had composite fillings. He also came away convinced of the superior durability of amalgam, he said.
"The composites failed at a much higher rate," Dr. Martin said. "We concluded amalgam should remain a clinical option."
No final conclusions
The panel did not vote on final conclusions, but several members stressed their wish to know more about the effects of mercury on certain subpopulations.
Joel M. White, DDS, MS, a professor in the division of biomaterials and bioengineering at the University of California, San Francisco, said he believed there is "clearly a subgroup of highly sensitive individuals where amalgam is contraindicated."
"I want to know that subpopulation, that subgroup, and where that threshold is," he said. "That will make me a better dentist and patients more trusting of dentists and the FDA."
Dr. White asked if any of the consumer groups who petitioned for the meeting had developed a composite study that might help researchers better identify people likely to suffer adverse reactions to mercury.
David Kennedy, DDS, who testified on behalf of the consumers, said no such study existed. But, he added, "You are asking a question that should have been asked 50 years ago."
Following the conclusion of the meeting, the ADA praised the panel's finding that the FDA acted appropriately when it ruled last year that dental amalgam is a safe and effective treatment option for the general population.
"The panel recommended continuing review of existing and new scientific information as it becomes available," said ADA President Raymond Gist, DDS, in a press release. "As with all clinical issues, our position on amalgam is based on the best available science. We will continue to maintain or revise our positions on oral health and oral healthcare issues accordingly. At the end of the day, all treatment decisions should be made by patients with the advice of their dentists. We support the rights of all patients to decide how best to maintain and improve their oral health."
Copyright © 2010 DrBicuspid.com