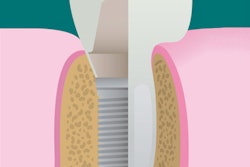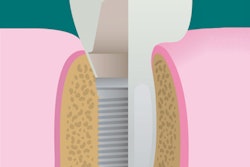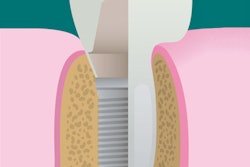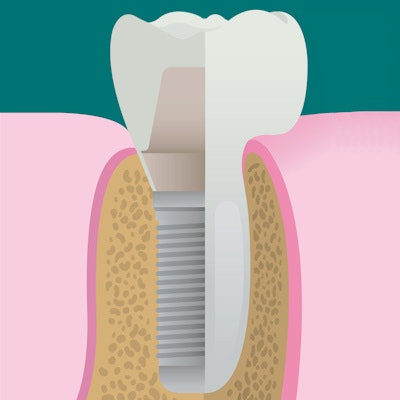
A newly designed porous titanium-silica composite implant may provide a solution to the infectious complications that can plague dental implant patients, according to a new study. Researchers have developed a device with an internal reservoir that can be loaded with antimicrobial compounds that are released over time.
The effectiveness of a continuous release of chlorhexidine at preventing and eradicating Streptococcus mutans biofilm formation was shown in the laboratory by researchers from Belgium.
"We provide a proof of concept of the sustained release of an antimicrobial compound from an internal reservoir through the titanium/silica material to the implant outer surface and surroundings," the study authors wrote in European Cells and Materials (January 2017, Vol. 33, pp. 13-27). "Release of this antimicrobial compound prevents and eradicates microbial biofilm formation on the rough implant surface, decreasing the risk for development of peri-implant diseases."
The study authors are from the University of Leuven. They were led by Kaat De Cremer, a postdoctoral researcher at the university's Centre of Microbial and Plant Genetics, and Annabel Braem, PhD, a postdoctoral researcher in the department of materials engineering.
Panacea for roughened surfaces
Bacteria can form biofilms on dental implants, which can trigger infection and inflammation in the peri-implant tissue and lead to peri-implant bone loss. Implants with roughened surfaces used in recent years to improve osseointegration may facilitate biofilm formation because of an increase in total surface area, according to the authors. New strategies are needed for preventing biofilm formation, they noted.
“This antimicrobial compound prevents and eradicates microbial biofilm formation on the rough implant surface, decreasing the risk for development of peri-implant diseases.”
With this is mind, the researchers developed an implant that combines the high strength of a macroporous titanium structure with the drug-release functionality of a mesoporous silica material. This design allows a continuous diffusion of antimicrobial compounds from the device's internal reservoir, which can be reloaded. The amount of the compound released can be modified by adjusting the concentration of the feed solution.
In the current study, the authors wanted to show proof of concept for the device. They used chlorhexidine, as it is one of the most commonly used and best-documented antimicrobials in dentistry. However, other antibacterial compounds, including antibiotics, could be inserted, as well as multiple compounds simultaneously.
"It is hypothesized that diffusion of antimicrobial compounds through the bulk of the implant from the refillable reservoir can establish a stable continuous release without burst effects over a prolonged period of time, effectively preventing biofilm formation during a long time frame," the authors wrote.
Using an in vitro test tool they had previously developed, the researchers conducted experiments on the release of chlorhexidine from the titanium-silica composite material they developed that was shaped into disks. They demonstrated the release of chlorhexidine over a 10-day period using a 5-millimolar (mM) chlorhexidine solution in the implant reservoir.
Then the researchers assessed if the system could prevent biofilm formation in vitro. They found that the metabolic activity of S. mutans biofilm cells was significantly decreased (± 99% reduction) with multiple chlorhexidine feed concentrations compared with feed solutions without it.
To show that their system could treat established infections, they allowed S. mutans biofilms to grow on the release side of the titainium-silica disks for two days in the absence of chlorhexidine. Then they administered a 0.25-mM chlorhexidine solution through the feed compartment and found that it also significantly reduced metabolic activity (± 98% reduction) of S. mutans after two days.
Next, the researchers tested chlorhexidine release in vitro using the dental implants they designed. They found significantly reduced metabolic activity (± 99% reduction) of S. mutans biofilm cells on implants that released chlorhexidine compared with control ones that didn't.
They also examined the cytotoxic effects of chlorhexidine on MG63 osteoblast-type cells. Osteoblasts are integral to establishing and maintaining implant osseointegration, and there is evidence that chlorhexidine may have deleterious effects on various cell types in vitro. In the current study, the researchers found that chlorhexidene at concentrations exceeding 1 µM (approximately 0.00006%) directly applied to osteoblasts was cytotoxic.
"The direct application of chlorhexidine at higher concentrations during regenerative therapy for the treatment of per-implantitis could thus have serious toxic effects on alveolar osteoblasts," the authors wrote.
Avoiding toxic effects
The authors acknowledged that the use of chlorhexidine mouthwash (0.12%) after implant installation is routine, and that a study found that patients irrigating with diluted chlorhexidine (0.06%) using a powered oral irrigator with a special subgingival irrigating tip showed reduced infection symptoms compared with patients rinsing with 0.12% chlorhexidine gluconate once daily.
"These results suggest that tackling the zone most proximal to the implant results in superior health outcome," they noted. "This is precisely what is aimed for with the dental implant presented in this study, as it is capable of releasing chlorhexidine directly at the implant surface."
The study results suggested that very low doses of an antibacterial compound are adequate when administered locally during a limited time, but the researchers were not able to repeat the experiments in the current study at a lower concentration of chlorhexidine. Nevertheless, they hope to evaluate less cytotoxic compounds with their dental implant device in follow-up experiments.
"Responding to the current need for more personalized treatments, our flexible implant design allows for the use of a broad range of antimicrobial compounds (alone or in combination), and fine-turning of the concentration at which these are released will depend on the patient's need," the authors concluded.