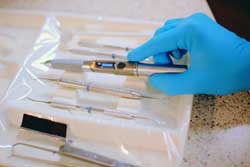
The FDA has formally accepted for filing Ondine Biopharma's premarket approval (PMA) application for the Periowave photodisinfection system for treating chronic periodontitis in adults as an adjunct to standard methods of care, the company announced. The FDA's action means that the PMA application is sufficiently complete and ready for substantive review.
"The acceptance of the PMA submission is a welcome step forward for Ondine and our business partner, Periowave Dental Technologies," said Carolyn Cross, chairman and CEO of Ondine, in a press release. "One of Ondine's key priorities is to obtain regulatory clearance in the U.S. for the marketing of Periowave, the first commercial application of the company's photodisinfection technology."
Periowave is a nonantibiotic photodisinfection system for the dental market developed by Ondine that uses a low-intensity diode laser and a wavelength-specific, light-activated photosensitive compound which, when used in conjunction with standard methods of dental care, reduces the symptoms of chronic periodontitis, according to the company.
Copyright © 2010 DrBicuspid.com