The anti-inflammatory drug dimethyl fumarate (DMF) may help repair cell energy systems and restore immune balance in the gums to reverse damage from periodontitis, according to a news release dated April 28.
Furthermore, DMF may help fight gum disease by regulating the cell cleanup process of Tu translation elongation factor (TUFM)-controlled mitophagy, which plays an important role in maintaining normal mitochondrial function, according to the release.
“DMF’s ability to fine-tune macrophage polarization through mitophagy is a game-changer in periodontal therapy,” Dr. Shengbin Huang of Wenzhou Medical University and lead author of the study, said in the release.
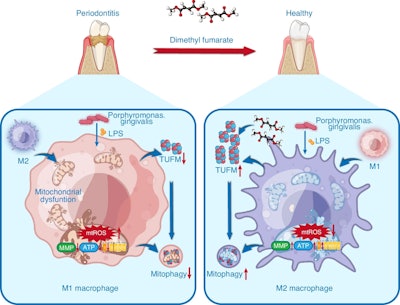 Schematic diagram for the beneficial effects of dimethyl fumarate against periodontitis through the regulation of TUFM-dependent mitophagy. Images and captions courtesy of Huang et al. Licensed under CC-BY-4.0.
Schematic diagram for the beneficial effects of dimethyl fumarate against periodontitis through the regulation of TUFM-dependent mitophagy. Images and captions courtesy of Huang et al. Licensed under CC-BY-4.0.
An overactive inflammatory immune response, especially from certain immune cells called macrophages, worsens gum disease. Problems with cell energy production and oxidative stress may make it harder for these cells to shift from causing inflammation (M1) to supporting healing (M2). New research is focusing on improving mitochondrial function and balancing the immune system to treat periodontal disease, according to the release.
Animal and lab-based models were used to investigate how DMF affects periodontal disease. Mice with induced gum disease treated with DMF showed significantly less bone loss and inflammation. Imaging techniques like immunofluorescence and micro-CT revealed improved bone density and fewer bone-resorbing cells.
In cultured macrophages, DMF reduced pro-inflammatory markers and increased healing markers. It also helped restore healthy cell function by improving mitochondrial energy levels and reducing oxidative stress. A key part of this effect involved TUFM, a mitochondrial protein that DMF protected from degradation, which supported mitophagy and mitochondrial balance, according to the release.
When TUFM was blocked, DMF’s protective effects disappeared, showing how important this pathway is. Interestingly, DMF was even more effective than the antioxidant MitoQ in improving immune balance and cell health.
Since DMF is already approved for other conditions, it may reach clinical use for gum disease more quickly. Future treatments might use DMF in a hydrogel form to target the gums directly while reducing effects on the rest of the body, according to the release.
“These insights could redefine how we treat chronic inflammatory conditions beyond the oral cavity,” Huang said.