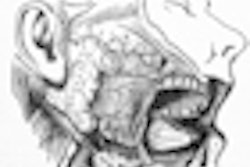
Sosei has acquired development and commercialization rights to Loramyc in Japan from BioAlliance Pharma. Loramyc is an antifungal agent administered as a mucoadhesive buccal tablet for treating oropharyngeal candidiasis in immunocompromised patients.
BioAlliance received its first Loramyc marketing authorization in France in October 2006. Loramyc has since been registered in 26 European countries, South Korea, and the U.S.
Under the terms of the agreement, Sosei will pay BioAlliance an upfront license fee of $3 million and further development and sales-based milestones totaling up to a maximum of $18.5 million. Sosei will also pay royalties on net sales of the product.
Loramyc has the potential to become the first treatment in tablet form for oropharyngeal candidiasis in Japan, according to Shinichi Tamura, CEO of Sosei Group.
Oropharyngeal candidiasis is commonly found in immunocompromised patients, including HIV and cancer patients, and in other chronic disease states such as diabetes. It is the most frequently occurring infection in head and neck cancer patients undergoing radiation therapy.