Failing to take care of oral health may result in more than cavities and other dental problems. Inflammatory bowel disease (IBD), which includes ulcerative colitis and Crohn's disease, may be linked to poor oral health, according to an animal study published on June 16 in Cell.
Disease-causing oral bacteria, such as those associated with periodontitis, worsened intestinal inflammation in mice with inflammatory bowel disease, the authors wrote.
"The oral and gastrointestinal mucosae are microbiologically and immunologically connected," noted the group, led by Sho Kitamoto, PhD, from the department of internal medicine at the University of Michigan.
Prior research has shown a connection between oral health and systemic diseases. Periodontitis has been linked to hypertension, heart attacks, and strokes, and poor oral health has been connected to type 2 diabetes and pregnancy complications.
How oral infection contributes to the development of other diseases remains unclear, but scientists continue to find links, including the latest to IBD. Approximately 3 million people in the U.S. have been diagnosed with inflammatory bowel disease, which causes chronic inflammation in the gastrointestinal tract. The exact cause of IBD is unknown and a cure has yet to be found.
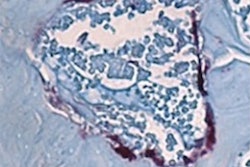
In the current study, 8- to 12-week-old male and female mice were used to examine how oral health affects gut health. Ligatures were placed around some of the animals' teeth to facilitate local accumulation of bacteria to enhance bacteria-mediated inflammation and bone loss. Two weeks after oral ligation, the mice were treated with dextran sodium sulfate to induce experimental colitis.
The colitis-induced mice had significantly increased body weight loss, higher disease activity, and more inflammation in the colon than the controls, Kitamoto and colleagues found.
Periodontitis creates an imbalance in the oral microbiome and leads to an increase in the number of pathogens, including Klebsiella and Enterobacter species, which then travel to the gut, causing inflammation. However, oral pathogens didn't populate the gastrointestinal tract of those with healthy colons, according to the authors.
To better understand the immune response of the mice, Kitamoto's group measured the characteristics of their cells using a cytometry by time-of-flight (CyTOF) panel. Mice given dextran sodium sulfate had dramatically increased myeloid and lymphoid infiltration, and oral ligature treatment alone also enhanced immune infiltration into the gut lamina propria, including T helper 17 (Th17) subsets, B cells, and gamma delta (γδ) T cells. Periodontitis activated the immune system cells Th17 and Th1, which exacerbated gut inflammation.
Inflammatory bowel disease creates a perfect environment for oral bacteria: When the colon is inflamed due to IBD, its healthy gut bacteria are disrupted, making it more difficult for the gut to resist disease-causing oral bacteria, according to the authors.
The study had no limitations, and the results suggest a potential way to reduce the risk of developing IBD.
Ensuring that patients maintain optimal oral care by removing disease-causing oral biofilms and reducing periodontal inflammation may reduce IBD risk, according to the authors. This may restrict pathogenic effector T lymphocytes and their reactive antigens from expanding.
"Likewise, inhibition of migration of oral [effector memory T cells] to the gut might be an alternative strategy used to block the pathogenic mouth-gut axis in the context of IBD," they wrote.