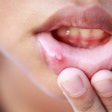
In the quest to better understand the causes of periodontal disease, researchers are making big strides on two key fronts: understanding the nature of the bacteria that stimulate gingival inflammation, and the genetic and physiologic foundations that can determine the body's response to that stimulus.
Inflammation key to understanding periodontal disease
As a common denominator for a broad range of medical conditions, inflammation has become an especially hot topic in periodontal research. Study after study has set out to try and understand why some people have an inflammatory response to certain bacteria and others don't, according to Pamela McClain, DDS, president of the American Academy of Periodontology (AAP).
"Our understanding of periodontal disease is veering away from what was considered to be just bacteria causing the disease to the role of inflammation, which is believed to be the most important factor in the progression of the disease," she explained. "That response can truly vary from one individual to the next. You can have a husband and wife with similar bacteria in their mouths -- and studies show they commonly do -- yet one gets inflammation and the other doesn't. We know the bacteria start the process, but it's the response to those bacteria that ultimately results in the loss of the attachment between the bone and the periodontal ligament."
Genetic factors
Genetics is one of the most significant factors in that response. Genetic factors ranging from gender -- more specifically, being female -- to carrying some distinctive single nucleotide polymorphism are believed to play a role in 30% of periodontal disease cases. In two separate studies, Alexandre Vieira, DDS, PhD, and colleagues at the University of Pittsburgh found evidence that genes could, in fact, account for as much as 50% of an individual's susceptibility to developing caries or periodontitis.
In the first study, Dr. Vieira and colleagues in Pittsburgh and Brazil analyzed 389 individuals in 76 nuclear families and found an association between two variants of the gene FAM5C and periodontal disease (PLoS One, April 7, 2010, Vol. 5:4, p. e10053).
In the second study, published in the Journal of Dental Research (June 2010, Vol. 89:6, pp. 631-636), Dr. Vieira and his co-authors found that people with a variant of the gene DEFB1 (defensin, beta 1) were more than five times more likely to have decayed, missing, or filled teeth compared with those who didn't carry the variant. Patients with another variant, the rs179946 (G-52A), were only a third as likely to have the dental problems (p = 0.014).
Interestingly, the FAM5C gene has also been associated with another inflammatory condition: heart disease.
"There are a number of common etiological factors modulating these diseases," said Dr. Vieira, an associate professor and the director of clinical research at the University of Pittsburgh Center for Craniofacial and Dental Genetics. "It appears that the association between these two groups of diseases could be in part related to individual genetic background."
The same genes modulate inflammation regardless of the process that is happening in the oral cavity or elsewhere, he added.
"Individual responses to specific external factors, since they are modulated by the same genes, can be similar, and this can partly explain associations reported between periodontal diseases and other systemic conditions," he said.
Other experts agree that the discovery of similarities between the inflammatory process in periodontal disease and that of other chronic inflammatory diseases could represent one of the most promising areas of research.
"It is uncanny how the inflammatory reaction occurs in other chronic inflammatory diseases and periodontitis," said Samuel Low, DDS, a past president of the AAP and a professor of periodontology at the University of Florida College of Dentistry. "With diabetes, cardiovascular disease, Alzheimer's disease, and rheumatoid arthritis, for instance, if you review the way the inflammatory process works in those diseases, it is very close to the way it works with periodontal disease."
One important advantage that periodontal disease has over the other inflammatory conditions, Dr. McClain noted, is a clearly defined stimulus: bacteria.
"With rheumatoid arthritis, the body is attacking the joints with inflammation. Likewise with periodontal disease, the inflammation is in the attachment between the bone and the attachment of the gum tissue," she said. "But at least with periodontal disease, we know the bacteria are stimulating this whole response. In rheumatoid arthritis or other conditions, we often don't know."
New bacteria insights
That being said, more headway is being made in understanding the behavior of the bacteria itself.
Intriguing new research on one of the most notorious of periodontal disease offenders, Porphyromonas gingivalis, implicates the bacterium as a "keystone pathogen" that, in fact, doesn't directly cause damage, but instead manipulates the oral environment so that otherwise benign bacteria change course and infect the tooth's supportive structures (Journal of Oral Biosciences, 2011, Vol. 53:3, pp. 233-240).
"In this regard, P. gingivalis' tactics to undermine innate immunity may promote the survival of other members of the periodontal biofilm community," wrote the study authors, from the University of Louisville School of Dentistry.
Based on observations on mice, the researchers found that P. gingivalis, in effect, reprograms the front-line immune cells that protect the space in the subgingival crevice, causing them to let down their defenses. Once that's accomplished, benign bacteria -- and not P. gingivalis -- then rise in numbers and march in to infect the tooth's periodontium.
"These subversive strategies of P. gingivalis may explain, at least in part, its ability to persist and establish chronic infections in the periodontium," the authors wrote.
Interestingly, in a previous study, researchers with the University of Michigan found that people carrying an antibody to a protein of P. gingivalis, called HtpG, have a lower risk of periodontal disease (PLoS One, April, 23, 2008, Vol. 3:4, p. e1984). The antibody "offers significant potential as an effective diagnostic target and vaccine candidate," the authors concluded.
Nongenetic causes
Genetics holds plenty of clues to the causes of periodontal inflammation and disease, but it doesn't paint the whole picture. Other well-known risk factors include everything from smoking to pregnancy (and the state of hormonal flux that it involves), but one key condition is looming ever larger as an important factor in periodontal disease: obesity.
Research demonstrating the role of being overweight and obesity in periodontal disease has mounted in recent years, with one of the more interesting studies coming out of the Case Western Reserve University School of Dental Medicine (Journal of Periodontology, October 20, 2011). The study authors found significant improvements in periodontal health among gastric bypass patients following their surgeries and weight loss.
The study included 30 obese people with chronic periodontitis. The researchers compared the participants before and after half of the subjects underwent bypass surgery and had fat cells removed from the abdomen. Compared with those who did not have the weight loss surgery, the bypass patients showed greater improvements in periodontal attachment, bleeding, probing depths, and plaque levels.
Importantly, the gastric bypass patients also showed declines in glucose levels following the procedure. The researchers theorized that by making insulin less resistant, weight loss improves diabetic status, which in turn improves response to periodontal treatment. They also speculated that the surgery reduced the production of the appetite hormone leptin, which has been implicated in the regulation of metabolism and may be linked to inflammation due to its role in increasing the production of cytokines and C-reactive protein.
Other studies also have shown evidence of weight loss resulting in reduced periodontal disease, and the combined research supports the suggestion of a relationship between the increased fat cells that occur with obesity and periodontal inflammation, Dr. Low said.
"Belly fat, interestingly enough, becomes an endocrine gland within itself, and with the increase of the fat cells, there is an overstimulation of the inflammatory process," he said.
Dr. Low noted that diabetes is the second-highest risk factor for periodontal disease, behind smoking.
The relationship between the two diseases is believed to be somewhat reciprocal, with gingival inflammation undermining blood sugar control while diabetes-associated high blood sugar in turn may trigger periodontal inflammation. But the fact that obesity is a leading cause of diabetes would appear to further compound the damage the disease can wage on periodontal tissue.
Prescription drugs
Other known culprits in the development of periodontal disease are medications. Prescription drugs known to potentially cause gingival ulcerations include aantihypertensive drugs, calcium channel blockers, and even some anti-inflammatories.
Some of the newer offenders include certain chemotherapy drugs, antidepressants, and antianxiety medications, which can all cause xerostomia, according to Dr. Low.
"There are probably more than 500 medications out there that create dry mouth," he said. "Antidepressants and antianxiety medications, for instance, can cause this, and the danger is that saliva is one of the best protectors against inflammation. It contains powerful anti-inflammatory products. So if you don't have saliva, you lose your natural defense system."
Certain medications can cause swelling of the gum tissues and result in pseudo or false pockets, making it easier for the disease to progress, Dr. Low added.
Xerostomia resulting from medications can trigger a particularly vicious cycle of erosion on the teeth and gums, Dr. McClain emphasized.
"When you have less saliva being produced, you're not washing away the bacteria or having the good enzymes that are necessary to fight the bacteria," she said. "That results in more plaque formation and a bigger challenge in managing periodontal issues."
Dental practitioners should stay informed of data on new drugs and new drug classes in the marketplace, Dr. McClain added. "We often don't know the effects of these medications on the teeth or gums until patients have been on them for a long time," she said.
The issue is tricky, however -- some medications may clearly be essential in treating a patient's medical condition, and Dr. Low warned dentists to use caution in advising patients on their prescription drugs.
"We recommend that dentists tell patients to ask their physician if there is an alternative medication if the current medication they are taking is risking periodontal health," he said. "If the patient's doctor says the condition is life-threatening and there is only one drug, then we'll deal with the dry mouth. But if we find that the dry mouth is contributing to either decay or periodontal disease, then it's important to see if there is an alternative."
Fatty acids: Bad and good
Aside from medications, the causes of periodontal disease may also simply lie in the foods patients consume. When it comes to the causes of inflammation, recent research has revolved around fatty acids, both bad and good.
Not surprisingly, many of the same fatty acids implicated in increasing bad cholesterol levels and potentially raising the risk of cardiovascular disease have been linked to periodontal disease.
In one recent study out of Japan, for instance, older nonsmokers who consumed higher levels of saturated fatty acids, including those found in meat fats, milk, butter, lard, and some oils, had a relative risk of periodontal disease that was 1.92 times higher than that of people of the same age with a low saturated fatty acid intake (Journal of Dental Research, July 2011, Vol. 90:7, pp. 861-867).
Conversely, just as polyunsaturated fats, including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are associated with a host of health benefits, ranging from cardiovascular to neurological, so too are the fats emerging as potential key players in the prevention of inflammation and disease.
One recent study involving more than 9,000 adults showed the prevalence of periodontitis to be approximately 20% lower among individuals who reported consuming the highest amount of dietary DHA; reduction, although smaller, was also seen with consumption of EPA (Journal of the Academy of Nutrition and Dietetics, November 2010, Vol. 110:11, pp. 1650-1652).
"We found that omega-3 fatty acid intake, particularly DHA and EPA, are inversely associated with periodontitis in the U.S. population," stated co-author Asghar Naqvi, MD, MPH, from Beth Israel Deaconess Medical Center, in a press release. "To date, the treatment of periodontitis has primarily involved mechanical cleaning and local antibiotic application. Thus, a dietary therapy, if effective, might be a less expensive and safer method for the prevention and treatment of periodontitis."
Another line of research on omega-3s adds that the inclusion of low-dose aspirin (81 mg) significantly improves the ability of omega-3 fatty acid supplementation to resolve inflammation.
In one study of 80 human subjects, including 40 who received scaling and root planing and 40 who also received the treatment in addition to supplementation with low-dose aspirin and omega-3 fatty acid (900 mg of EPA and DHA), significant reductions in probing depths and attachment gain were observed after three and six months in the supplementation group, compared with the control group (Journal of Periodontology, November 2010, Vol. 81:11, pp. 1635-1643).
"Omega-3- and omega-6-based polyunsaturated fatty acids play a major role as defense-supporting/catalyzing lipids. The problem is that humans (and many mammals) do not have the means to metabolize these lipids and cannot produce them in sufficient quantities," said Alpdogan Kantarci, DDS, PhD, of the Forsyth Institute and co-author on several key studies examining the issue. "What we produce is very limited, and their half-lives are very short. Therefore, we need to consume external sources such as fish or plants."
Aspirin prolongs the half-life of such compounds in blood and dampens the inflammatory reactions by counteracting the mediators and cells that are produced as the first line of defense against pathogens, he added.
"The more we appreciate these mechanisms, the more targeted our treatment would become in inflammatory diseases, including the periodontal diseases," he said.
In a previous study on mice and rabbits (Journal of Immunology, November 15, 2007, Vol. 179:10, pp. 7021-7029), the researchers even found that omega-3 benefits appeared to extend to the bones, Dr. Kantarci said.
"We have shown that such an approach cannot only reverse the inflammatory process, but also can help in regenerating the lost bone and hard tissues, so there is a lot of exciting potential," he said.
– Samuel Low, DDS
As more becomes known about the mechanism of omega-3 fatty acids, the more valuable they may become as a nonpharmaceutical approach in the prevention or treatment of inflammatory diseases, including periodontal disease.
"This does not mean that the antibiotics will be replaced soon, since many infections will most probably still require elimination or decrease of bacterial load," Dr. Kantarci said. "But in complex infecto-inflammatory diseases, modulation of the host response can provide novel ways to control and treat the process."
Dr. Low already recommends that his patients at risk for periodontal disease take omega-3 supplements -- and low-dose aspirin.
"We're very excited about this because research has demonstrated that supplementation of 900 mg of omega-3 and 81 mg of aspirin can decrease periodontal disease," he said. "The omega-3s basically suppress bad inflammation and express good inflammation. We can also throw in calcium and vitamin D for the bones, but to me the more appropriate approach is anything that is anti-inflammatory."
Looking ahead
The periodontal community can likely expect to see much more intriguing research as the secrets of chronic inflammatory diseases unfold. Dr. Low recommends dentists simply stay tuned and follow the science.
"What we don't want to see is dentists falling back on anecdotal data," he cautioned. "The 'I've seen patients for 25 years and when I've seen this, I do that' approach is just not wise at this point in time. What we want is an approach that respects the scientific relationship between the risk factors, the genetic factors, and the progression of the disease."