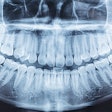
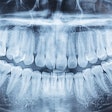
The U.S. Food and Drug Administration (FDA) has granted marketing clearance to the NewTom VG3 digital panoramic, cephalometric, and tomographic x-ray system.
The NewTom is manufactured by Cefla Dental; Biolase is currently the exclusive distributor in the U.S. and Canada.
The VG3 is a complement to the full field-of-view (FOV) provided by the company's NewTom VGi and will sell in the U.S. for $50,000 to $120,000, Biolase noted in a press release. It is a modular hybrid 2D/3D system that can start as a digital 2D panoramic x-ray system and can be upgraded to add either a cephalometric sensor and software, necessary for orthodontics and diagnosing head, jaw, and facial pain, and/or 3D cone-beam CT with an 11 x 8-inch FOV, used for implants, endodontics, gum disease, sinus problems, and oral and maxillofacial surgery.
The VG3 comes equipped with NewTom's NNT 4.0 imaging analysis software and incorporates NewTom's Safebeam technology, which emits the lowest possible dosage of radiation. Safebeam technology automatically adjusts the radiation dosage and continuously monitors systems operations, thereby eliminating the possibility of unnecessary exposures.