Vatech America has received U.S. Food and Drug Administration (FDA) clearance for PaX-Flex, a field-upgradable digital x-ray system, the company announced.
The PaX-Flex is a single-platform system that is capable of possessing multiple modalities and can be customized and upgraded before and after the initial purchase, according to Vatech.
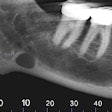
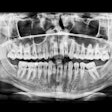
Because the system is equipped with CMOS digital sensors, panoramic x-ray images can be captured in about 10 to 18 seconds. In addition, the PaX-Flex features autofocus technology to produce optimal digital diagnostic images using Vatech's proprietary adaptive layer mode panoramic tomography solution.
Cone-beam CT also can be added to the PaX-Flex, allowing users to view a 3D radiograph captured in desired fields-of-view ranging from 5 x 5 cm to 8 x 5 cm. Featuring 0.20-0.12 voxel sizes and 0.5 focal spot, images should be clear and easy to interpret, the company said. Included imaging software helps guide the user through case diagnosis and treatment planning.