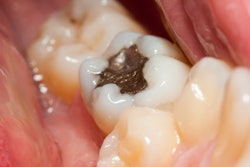
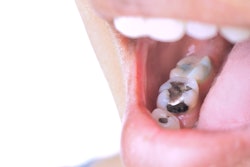
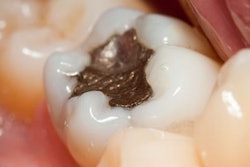
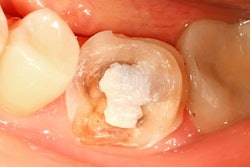
Instead of recommending a complete ban of dental amalgam, the U.S. Food and Drug Administration (FDA) is advising that high-risk individuals, such as pregnant women, avoid getting these fillings "whenever possible and appropriate."
Those considered high risk are advised to receive nonmercury materials, such as composite resins or glass ionomer cement fillings. Previously, the agency warned that people in these groups may be at greater risk of adverse health events from mercury-containing fillings and suggested they talk to their dentists about alternatives, but the FDA stopped short of recommending they "avoid" them.
Though the agency maintains that the "majority of evidence suggests exposure to mercury vapor from dental amalgam fillings doesn't lead to harmful health effects for most people, there may be some effects in people with certain health issues," it said in a statement issued on September 24.
The FDA listed the following groups as high risk:
- Pregnant women and their developing fetuses
- Women who plan to become pregnant
- Nursing women and their newborns and infants
- Children, especially those younger than 6 years
- People with preexisting neurological diseases, such as multiple sclerosis, Alzheimer's disease, or Parkinson's disease
- People with impaired kidney function
- People with known heightened sensitivities or allergies to mercury or other dental amalgam components
This is also the first time the FDA has listed multiple sclerosis, Alzheimer's disease, and Parkinson's disease in its guidance.
The FDA made these recommendations after evaluating published literature and considering comments from healthcare officials and the public that were received at the end of 2019. Some expressed concern about the cumulative effect on underserved communities of mercury vapor exposure from dental amalgam.
Many factors and uncertainties surrounding how much mercury vapor is released from dental amalgam affected the current decision. The amount of vapor released can depend on a filling's age, as well a person's habits, such as teeth grinding. Also, there are still uncertainties about the following:
- The effects of long-term exposure to dental amalgam on the high-risk groups
- The potential for mercury in dental amalgam to convert to other mercury compounds in the body
- Whether the accumulation of mercury in some bodily fluids and tissues results in other unintended health outcomes
Despite the update, the agency still does not recommend removing or replacing existing amalgam fillings that are in good condition unless it is medically necessary.
In related news, though the International Academy of Oral Medicine and Toxicology (IAOMT) applauds the agency's recommendation for high-risk individuals, its representatives believe it's not enough.
"This is certainly a step in the right direction," Jack Kall, DMD, IAOMT executive chairperson of the board, said in a statement issued on September 25. "But mercury shouldn't be placed in anyone's mouth. All dental patients need to be protected, and dentists and their staff also need to be protected from working with this toxic substance."
The academy, which has lobbied for tougher restrictions for about three decades, has compared the delay in safety regulations for dental amalgam with those that occurred with cigarettes and lead-based products.
"Over 45% of dentists worldwide are still estimated to be using amalgam, including a large majority of dentists for military and welfare agencies," David Kennedy, DDS, a member of the IAOMT board of directors, said. "It should not have taken 35 years to get to this point, and [the] FDA now needs to protect everyone."