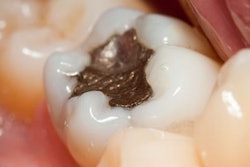
Dental amalgam is safe for patients and clinicians, said scientists from the U.S. Food and Drug Administration (FDA) during a public meeting on November 13 that aimed to discuss the efforts being made to evaluate dental amalgam and metal-containing implants.
No new published evidence would suggest considerable amalgam-related risks for the U.S. general population or dental professionals, said Lisa Torosyan, MD, PhD, a health scientist at the FDA, during the meeting held in Gaithersburg, MD.
"The current risk of considerations is based on the absence of strong evidence on amalgam attributable adverse outcomes, not on the presence of evidence that would eliminate such possibilities," Dr. Torosyan said.
The FDA and members of the public were given opportunities to discuss dental amalgam and the immune and inflammatory reactions patients experienced, during an Immunology Devices Panel of the Medical Devices Advisory Committee meeting.
Healthcare professionals and others interested in informing the agency's thinking on the topic are encouraged to submit written comments online. The agency will not accept comments after December 13.