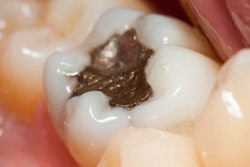
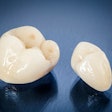
The U.S. Food and Drug Administration (FDA) will host a public meeting in November to discuss ongoing efforts to evaluate dental amalgam and metal-containing implants and to address potential safety questions.
The Immunology Devices Panel of the Medical Devices Advisory Committee meeting will be held on November 13-14 in Gaithersburg, MD, and the public can attend in person or online.
"The panel will be looking at the potential for certain patients who receive medical device implants that contain select metal or metal alloys to develop immune and inflammatory reactions and whether current approaches and standards for biocompatibility are adequate," according to a statement issued on September 30 by Jeffrey Shuren, MD, JD, the director for tthe FDA's Center for Devices and Radiological Health.
To better understand how a patient responds to materials used in medical device implants and harness that information to improve the safety of devices, the FDA is working to engage scientists, patients, healthcare providers, and industry stakeholders to determine the following:
- The current state of the science
- The critical gaps in the existing science
- What approaches should be considered
The FDA's discussions will include two of its recently released paper on the topics.
The metal-containing implants paper, "Biological Responses to Metal Implants," summarizes the science of the human body's local and systemic responses to the most common metals present in implantable medical devices. Also, it addresses gaps and opportunities for further research on the topic.
The dental amalgam paper, "Epidemiological Evidence on the Adverse Health Effects Reported in Relation to Mercury from Dental Amalgam: Systematic Literature Review (2010-Present)," assesses existing medical studies related to neurological, cardiovascular, developmental, and other outcomes in populations exposed to dental amalgam.
Those interested in informing the agency's thinking on the topic are encouraged to attend the meeting or submit written comments online. Comments submitted by October 10 are forwarded to the committee. The FDA will still consider those received after that date. The agency will not accept comments after December 13.
Though the agency recognizes that most patients using medical devices do not have adverse reactions, the FDA remains committed to improving patient treatment options and outcomes for all.
"We are interested in stakeholders' perspectives on our recent review, as well as any new information that they may have to share, to help inform our understanding of the current benefit-risk profile of dental amalgam," Dr. Shuren stated.