An iron-containing nanoparticle successfully disrupted pathogenic biofilms and prevented tooth decay without harming surrounding oral tissues or biofilms in a new study. The findings potentially open the door for developing a precise therapeutic approach for caries.
Researchers tested ferumoxytol, a U.S. Food and Drug Administration (FDA)-approved treatment for iron deficiency, and reported success at disrupting the biofilm that leads to caries. Their study was published on July 31 in Nature Communications.
"We used plaque samples from caries-active subjects to reconstruct these highly pathogenic biofilms on real human tooth enamel," stated study author Hyun Koo, DDS, PhD, in a University of Pennsylvania release. "This simulation showed that our treatment not only disrupts the biofilm but also prevents mineral destruction of the tooth's surface."
Dr. Koo is a professor in the department of orthodontics in the divisions of pediatric dentistry and community oral health at the University of Pennsylvania School of Dental Medicine in Philadelphia.
Biofilm-specific therapy
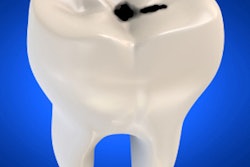
Dental caries is a biofilm-induced disease, and current antimicrobials and clinical modalities have limited killing activity against biofilm cells. New developments have led to the concept of activating substances in situ to target the harmful structural and biological traits of biofilms without damaging the surrounding tissues.
This concept led the researchers to examine the potential use of experimental iron oxide nanoparticles for this disruption. To see how ferumoxytol affected Streptococcus mutans, a well-characterized biofilm-forming strain, they added the nanoparticle to an actively growing S. mutans and propidium iodide culture. The group used high-resolution fluorescence imaging to see how effective the nanoparticle was at eliminating the bacteria.
“Microbiome and histological analyses show no adverse effects on oral microbiota diversity and gingival and mucosal tissues.”
The researchers also assessed whether daily topical treatments with the nanoparticle were able to disrupt cariogenic biofilm development and prevent enamel surface damage using an ex vivo biofilm model.
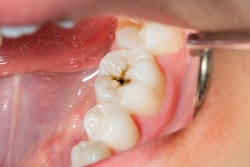
Plaque-biofilm samples were collected from children between the ages of 36 months and 72 months who were diagnosed with severe early childhood caries. The samples were then grown on sterilized human enamel blocks for 115 hours.
To test topical treatment regimen, the enamel blocks were treated twice-daily by placing them in ferumoxytol for 10 minutes. After each treatment, the biofilms were dip-washed and transferred to fresh culture medium. The blocks were removed at 115 hours for three-dimensional structural analysis.
In the S. mutans analysis, the fluorescence images show that S. mutans viability was affected as early as 10 minutes by ferumoxytol.
For the enamel blocks, researchers compared control and test (treated with ferumoxytol) groups of the plaque-biofilm samples. They observed large areas of enamel surface demineralization in the control group, while the test group was "essentially devoid of such opaque demineralized areas," the researchers reported.
In addition, some of the enamel surfaces from the control group had eroded, forming microcavities. Overall, the control group blocks' surface roughness was about 10 times greater than those of the test group. The test group's enamel surface was mostly intact and smooth, they noted.
"Collectively, these findings reveal that [ferumoxytol] can potently disrupt the development of cariogenic biofilms and prevent localized demineralization and caries-like lesions on tooth-enamel surface," the study authors wrote.
Suppressing development
The authors listed no study limitations.
However, they did note that the idea that the major public health issue of caries could be addressed by the inclusion of iron oxide nanoparticle-based therapy is attractive.
"Topical oral treatment with ferumoxytol and H2O2 [hydrogen peroxide] suppresses the development of dental caries in vivo, preventing the onset of severe tooth decay," the authors concluded. "Microbiome and histological analyses show no adverse effects on oral microbiota diversity and gingival and mucosal tissues."