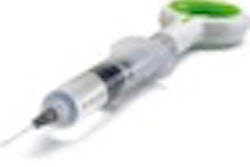
The U.S. Food and Drug Administration (FDA) has granted 510(k) marketing clearance for the Vatea endodontic irrigation device by ReDent Nova to accompany its self-adjusting file (SAF).
The device already has European Union CE Mark certification, according to Globes Online, adding that ReDent has also obtained the CE Mark for the head assembly of the SAF motor.
Copyright © 2010 DrBicuspid.com