Biolase Technology has issued a statement in response to the lawsuit by Discus Dental and Zap Lasers, arguing that its new iLase handheld laser for dentistry doesn't violate the other companies' patents.
Discus and Zap announced March 7 that they had filed suit in Los Angeles, accusing Biolase of patent and trademark infringement and unfair competition, and seeking monetary damages and an injunction.
Responding with its own statement, Biolase argued that the iLase is significantly different from the products offered by Zap and Discus.
In March 2010, Biolase announced that it had received 510(k) clearance from the U.S. FDA to market the iLase diode laser for dentists and hygienists to perform minimally invasive soft-tissue and hygiene procedures.
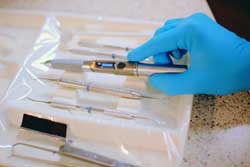
Biolase asserts that the iLase is the "first completely self-contained, handheld dental laser that includes the laser, user interface, battery power, and controls in a single, integrated handpiece with no foot pedals or cords."
"The plaintiffs' product is a diode laser with less than half the power, fewer features, of different physical design and size with a fixed angle tip versus our nine adjustable tips, and about double the price of the iLase," Biolase CEO David Mulder stated. "So we dispute their allegations, and believe the plaintiffs may be having difficulty competing with the iLase. ... In addition, while their product requires a cumbersome foot pedal to be used, the iLase is the only truly self-contained laser unit with no foot pedal."
Biolase also noted that the FDA asked Zap to recall 1,375 of its SoftLase, OrthoLase, and HygieneLase models in January.
"The SoftLase Lasers are in need of compliance upgrades due to lack of a remote interlock connector, an emission delay, user guide labels and locations, and calibration procedures and schedule," the FDA said.
"We look forward to our day in court," Mulder said, "and we are carefully exploring all options available to us, as the iLase has been in development since 2006. Our first iLase units are coming off the production line now, and we have been taking orders in the United States and around the world. As our European CE Mark was received prior to our FDA 510(k) clearance, most of the orders received to date are for international markets."
Copyright © 2010 DrBicuspid.com