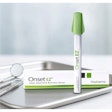
St. Renatus has secured a $5 million investment for its Kovacaine Mist anesthetic nasal spray, and will apply for FDA approval this year, having recently completed phase III clinical trials of the product.
The company has received a $5 million investment from Blue Ocean STR, an early investor, that will assist in the completion of the New Drug Application (NDA) submission to the FDA. The investment will also support St. Renatus' transition to a commercialization company to market Kovacaine Mist.
St. Renatus has completed all planned phase III clinical trials for the drug, and is preparing its data analysis for submission to the FDA for use on adult and pediatric dental patients without the use of a needle injection.
The spray targets the maxillary nerve, which controls nerves in the upper mouth, sinuses and nasal cavity. Kovacaine Mist uses a combination of the local anesthetic tetracaine and oxymetazoline hydrochloride, a topical decongestant used in nasal sprays.
In a phase II trial published last year (Journal of Dental Research, May 20, 2013), 25 of 30 patients given the spray and a placebo injection did not require further anesthetic during treatment. The recently completed phase III trials evaluated the spray in 380 adults, and an additional phase III trial tested it on 90 pediatric patients.
The St. Renatus name comes from the patron saint of anesthesia, René Goupil, and has Latin roots meaning "new beginning," according to the company.