Vigilant Biosciences has received International Organization for Standardization (ISO) 13485:2003 certification, an internationally recognized quality standard for medical devices.
The certification covers the design, development, production, sales, and service of Vigilant's OncAlert oral cancer-specific risk assessment products. This certification demonstrates that Vigilant has successfully implemented a quality management system that conforms to the worldwide standard for medical device and diagnostic manufacturing, according to the company.
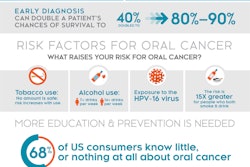
The Vigilant oral cancer risk assessment kit is formulated to detect proteins specific to oral cancer captured by an oral rinse. The test can be applied to every adult at risk, with particular emphasis on tobacco users, those who consume alcohol, and people with human papillomavirus (HPV). In a 300-patient study , the kit demonstrated the ability to detect tumors early and across a racially and ethnically diverse population.
Vigilant is an oncology company specializing in point-of-care and lab-based products that aid in the early detection and intervention of cancer.