Data from a Phase III clinical trial of 99m-Tc-tilmanocept, also known as Lymphoseek (Navidea Biopharmaceuticals) in oral and squamous cell cancers (SCC) suggest it may be improve surgical treatment of patients with head and neck SCC (HNSCC).
Researchers believe this is due to Lymphoseek's rapid transit through lymphatic vessels and targeted binding to the mannose binding receptors within key predictive nodes, according to a company press release.
A potential advantage of Lymphoseek is that substantially fewer lymph nodes are removed than with full nodal dissection, Navidea noted. This may enable surgeons to reduce the need for more extensive lymph node dissection, potentially sparing them from the morbidity and side effects of full nodal dissection while not sacrificing accuracy in the detection of key predictive lymph nodes.
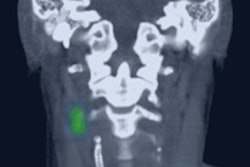
The Phase III clinical data were presented this week at the 65th Annual Cancer Symposium of the Society of Surgical Oncology meeting by Stephen Y. Lai, MD, PhD, of the University of Texas MD Anderson Cancer Center. Dr. Lai described the center's initial experience with Lymphoseek in sentinel lymph node mapping in nine patients with HNSCC.
All subjects received a single dose of Lymphoseek prior to undergoing surgery to remove some lymph nodes. Following surgery, all nine patients demonstrated a false negative of zero, the same as obtained with full nodal dissection, according to Dr. Lai.