Researchers have demonstrated a method of reversing bone loss and inflammation for patients with leukocyte adhesion deficiency (LAD).
Leukocyte adhesion deficiency is a rare but life-threatening disease. Patients can succumb to bacterial infections because their immune systems lack a molecule required by immune cells, neutrophils, to go to the site of infection.
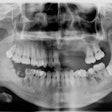
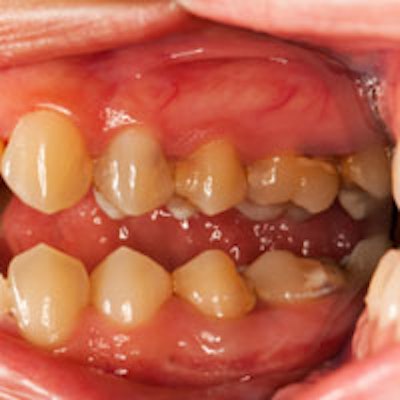
These patients often lose their teeth early in life, and the University of Pennsylvania School of Dental Medicine teamed with investigators from the National Institutes of Health (NIH) to help them.
The work was led by Penn Dental Medicine's George Hajishengallis, DDS, PhD, a professor in the department of microbiology, in collaboration with Niki Moutsopoulos, DDS, PhD, of the National Institute of Dental and Craniofacial Research.
Researchers used to believe that LAD patients developed severe periodontitis because of this inability of neutrophils to cross from the bloodstream into gingival tissue. Bacteria, therefore, were believed to thrive unchecked in the gingiva.
The view was so reasonable that nobody had questioned it, Dr. Hajishengallis explained, but the new study, published in Science Translational Medicine (March 26, 2014, Vol. 6:229, pp. 229ra40), challenges this assumption.
“Periodontal disease and bone loss continued unchecked even when LAD patients were given antibiotics or had their plaque removed.”
The team observed that periodontal disease and bone loss continued unchecked even when LAD patients were given antibiotics or had their plaque removed. As expected, the LAD patients did not have neutrophils in their gingival tissue, only in the bloodstream. Looking more closely, however, they realized that these patients had abundant bacteria on the surface of their teeth but normal bacterial levels inside their gingiva.
This was a very different form of periodontitis than what is seen in otherwise healthy people, in which the neutrophils can cause disease by being too active or present at high numbers in the gingiva.
To understand what was unique about the LAD patients' disease, the researchers examined their immune system-related genes and proteins. Compared with people with periodontitis or gingivitis who were otherwise healthy, one molecule in particular stood out: People with LAD had very high levels of IL-17 mRNA and IL-17-expressing cells in their gingival tissue.
These findings matched up with what the researchers had observed in mice that were bred to lack LFA-1, a molecule that normally helps neutrophils exit the bloodstream. Like human LAD patients, these mice had serious periodontal disease early in life, and their gingiva contained high levels of IL-17 mRNA and protein, as well as IL-23, a protein in the same pathway as IL-17.
IL-17 is in a signaling pathway that acts as a feedback loop for the immune system. When the pathway senses that a tissue contains few neutrophils, levels of IL-23 and IL-17 go up, encouraging more neutrophil production and migration from the bloodstream into the tissue. But, because LAD patients' neutrophils cannot move into the tissue, this pathway goes awry, leading to inflammation.
Not only can IL-17 encourage inflammation, but it can also encourage the development of osteoclasts, cells that break down bone, in this case teeth.
The researchers suspected that the bone loss was occurring because of IL-17 and not because the neutrophils could not control the bacterial infection.
To determine whether IL-17 or IL-23 was to blame for the periodontitis and bone loss in the mice with the LAD-like disease, the researchers injected molecules that block these proteins' activity in their gingiva.
They found out that blocking these two inhibited inflammation and bone loss, as well as the bacterial overgrowth. Periodontal bacteria thrive on inflammation, and consuming the breakdown products of tissues is how they get their food, Dr. Hajishengallis explained. Inhibiting inflammation starves them.
This result suggests that the reason bacterial numbers are so high in these mice, and, by extension, human LAD patients, is not because of a defect in the immune system's surveillance mechanism but because of the inflammation caused by the immune system's abnormal response to normal levels of bacteria in the gingiva, the study authors stated.
In other words, the bacterial overgrowth is the result and not the cause of this type of periodontitis, Dr. Hajishengallis noted.
Molecules that target and inhibit the activity of IL-17 are already used to treat autoimmune diseases such as psoriasis and rheumatoid arthritis, so the researchers want to see whether these compounds could also be used to treat periodontitis in LAD patients.