When oral bacteria join forces with fungi, they form cavity-causing "superorganisms." These oral health villains show unique resilience and mobility that make them more resistant to antimicrobials and lead to more tooth decay, according to a study in the Proceedings of the National Academy of Sciences.
More compelling, the researchers found that when Streptococcus mutans and Candida albicans cluster, it sprouts limblike structures, giving it unexpected surface mobility with coordinated "leapinglike" and "walkinglike" motions while continuously growing. This occurs despite each germ being unable to move on its own, the authors wrote. The researchers believe that this is the first time this group-level mobility has been reported.
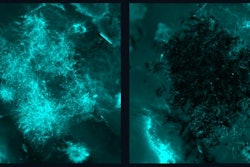
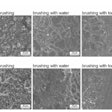
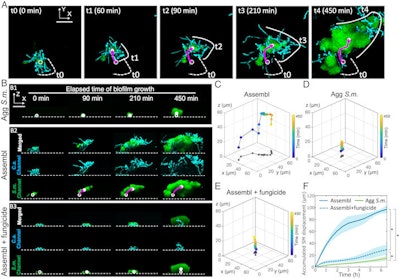 The dynamics of interkingdom group-level surface mobility and spreading. Images courtesy of Ren et al. Licensed by CC BY 4.0.
The dynamics of interkingdom group-level surface mobility and spreading. Images courtesy of Ren et al. Licensed by CC BY 4.0."These mobile groups of growing cells promote rapid spatial spreading of both species across surfaces, causing more extensive tooth decay," wrote the authors, led by Dr. Zhi Ren of the University of Pennsylvania in Philadelphia (Proc Natl Acad Sci, October 3, 2022, Vol. 119:41, e2209699119).
When fungi and bacteria connect, they form multicellular biofilms that cause many human infections. However, the level at which distinctive microbes act in concert with spatial and temporal qualities to coordinate disease-promoting functionality remains understudied.
To explore the dynamics of bacterial and fungal interactions in human saliva and how they develop biofilms on tooth surfaces, the researchers examined dental plaque and saliva samples from 14 caries-free children and 30 children with severe early childhood tooth decay. The research was funded in part by grants from the U.S. National Institute for Dental and Craniofacial Research, the European Research Council, and the Federal Ministry of Education and Research in Germany.
After seeing the bacterial-fungal clusters present in the saliva samples under a microscope, the researchers conducted experiments using real-time live microscopy to observe the process of attachment and eventual growth. To recreate the formation of these assemblages, the authors created a lab system using the bacteria, fungi, and a toothlike material, all of which were incubated in human saliva.
The platform enabled the researchers to watch the groupings merge. They observed a highly organized structure with bacterial clusters attached in a complex network of fungal yeast and filamentlike projections called hyphae. The structure was entangled in an extracellular polymer.
Additionally, the researchers tested the properties of these assemblages after they had colonized the tooth surface. They discovered enhanced surface adhesion, which made them very sticky, as well as greater increased mechanical and antimicrobial tolerance, the authors wrote.
Microbes with major moves
In these assemblages, the bacteria used the fungal hyphae to anchor on the surface and then propel the whole superorganism forward, transporting the attached bacteria across the tooth surface.
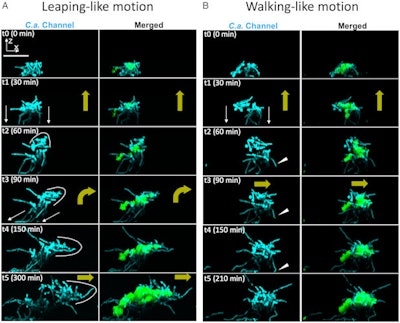 Assemblage mobility, bacterial hitchhiking, and spatial growth dynamics with a confocal image time series for growing interkingdom assemblages.
Assemblage mobility, bacterial hitchhiking, and spatial growth dynamics with a confocal image time series for growing interkingdom assemblages.When the bacteria and fungi teamed up, they moved quickly and at quite a distance. On the toothlike surface, the microbes' velocities were measured at more than 40 µm per hour. This velocity is like the speed of fibroblasts, which are cells involved in wound healing, according to the study.
Within the first hours of growth, the assemblages were seen leaping more than 100 µm across the surface. Though many questions remain concerning these mechanisms, researchers know that the germ collections' ability to move while they grow enables them to quickly colonize and spread to new surfaces. When the assemblages were allowed to attach and grow on human teeth in a lab model, more extensive tooth decay was discovered due to the biofilm spreading rapidly, they wrote.
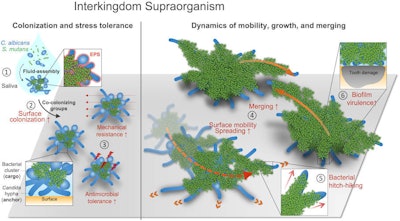 Interkingdom assemblages in human saliva behave like superorganisms with new functionalities and disease-promoting activity.
Interkingdom assemblages in human saliva behave like superorganisms with new functionalities and disease-promoting activity.Clinical significance
Nevertheless, the bacteria and fungi assembled as a new entity that gave each other different benefits and functions that they wouldn't have on their own, the authors wrote.
"These observations could be of clinical importance to provide insights into the onset of severe childhood caries characterized by rapid and aggressive decay of the tooth enamel," Ren and colleagues wrote.