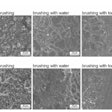
The U.S. Food and Drug Administration (FDA) has accepted an investigational new drug (IDN) application from C3 Jian for the company's synthetic peptide, designed to target Streptococcus mutans and other oral bacteria.
C16G2 is a synthetic peptide derived from C3 Jian's proprietary, pheromone signaling platform technology referred to as STAMPS (Specifically Targeted Antimicrobial Peptides). The indication identified in the new IND is for the use of C16G2 in preventing dental caries in adults, adolescents, and pediatrics.
C3 Jian expects to begin a phase I clinical trial that will enroll up to 36 subjects at New York University's (NYU) Bluestone Center for Clinical Research. Mark Wolff, DDS, professor and chair of the department of cariology and comprehensive care at NYU College of Dentistry, will serve as the principal investigator of the study.