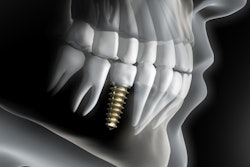
ClaroNav has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to expand its Navident EVO system for use in guided endodontics.
This technology is designed to provide minimally invasive access to tooth structures, helping dentists preserve tooth structures and save time when treating calcified or hidden canals, according to ClaroNav.