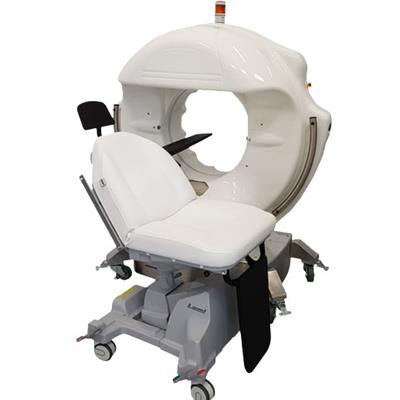
Robotic CT technology developer Epica has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its SeeFactorCT3 system, a multimodality mobile imaging scanner.
SeeFactorCT3 obtains high-resolution 3D volumetric CT images, as well as fluoroscopy and digital radiography (DR). The scanner also includes a detachable patient table and chair and sterile drape for interventional procedures. SeeFactorCT3 is designed for diagnostic and interventional use by physicians, surgeons, and dentists.
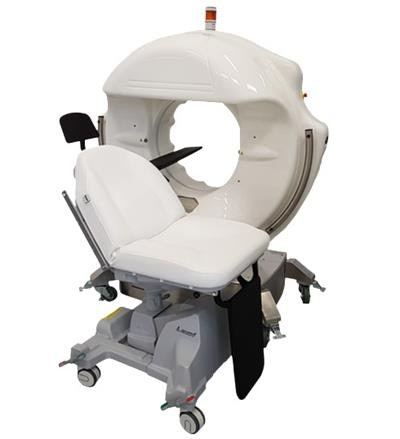 The SeeFactorCT3 scanner. Image courtesy of Epica.
The SeeFactorCT3 scanner. Image courtesy of Epica.The system delivers isotropic image resolution as fine as 0.1 mm in both soft and hard tissue, lesion detection as small as 0.2 mm, and Epica's Pulsed Technology to reduce radiation dose. Also, SeeFactorCT3 uses flat-panel digital detector technology that acquires sequences of the head, including the ear, nose and throat; dentomaxillofacial complex; teeth, mandible, and jaw; and temporomandibular joint.
SeeFactorCT3 represents Epica's move into clinical imaging from veterinary imaging, where the company makes imaging systems for use with animals. The company also plans to bring to market Medical Robot, which represents the integration of the company's CT imaging platform with a surgical-assist robotic arm.



















