The U.S. Food and Drug Administration (FDA) has given 510(k) clearance to Vivo Therapeutics’ oral appliances for treating severe obstructive sleep apnea (OSA) in adults.
Vivos’ removable CARE (complete airway repositioning and/or expansion) oral appliances, which include the DNA, mRNA, mmRNA devices, were cleared. The company reported that this is the first time that the FDA has granted clearance to an oral appliance to treat moderate and severe OSA in adults along with positive airway pressure and/or myofunctional therapy, as needed.
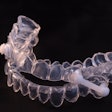
 The Vivos OSA device. Image courtesy of Vivos Therapeutics.
The Vivos OSA device. Image courtesy of Vivos Therapeutics.
Vivos’ appliances gradually reposition the hard and soft tissues that define the airway, thereby opening the airway and optimizing its function and flow. A study of 73 patients with severe OSA showed that 80% of patients experienced an improvement of at least one classification or at least a 50% improvement in the Apnea Hypopnea Index (AHI) after using the appliances, and 97% of patients improved or stayed the same, according to the company.