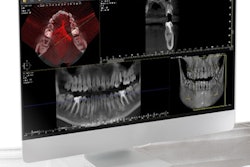
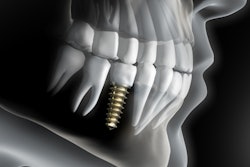
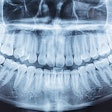
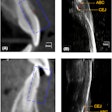
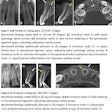
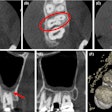
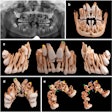
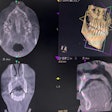
Owandy Radiology has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its I-Max 3D wall-mounted, panoramic digital radiography unit.
The unit includes multiple fields-of-view and four volume sizes. It is equipped with 3D software for case planning and can share stereolithography (STL) files from a dental lab or a digital scanner for CAD/CAM processes.
The unit weighs 145 lb, is recommended for endodontics with a 5 x 5-cm volume, and includes the company's automatic layers integration system for image quality and selection.
Owandy will feature the system at the upcoming Greater New York Dental Meeting.