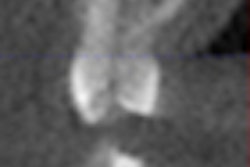
The U.S. FDA has issued a Class 2 recall of Zap Lasers' SoftLase Pro family of lasers, saying that the lasers are in need of compliance upgrades due to "lack of a remote interlock connector, an emission delay, user guide labels and locations, and calibration procedures."
In response, Zap Lasers sent a letter to customers stating that the SoftLase diode laser is eligible for a free compliance maintenance upgrade that will enhance the performance of the laser.
Customers are encouraged to call the firm's customer service line at 888-876-4547 to schedule their return merchandise authorization (RMA) number and shipping instructions. The Zap Lasers letter also lists the necessary upgrades.
Currently, 1,375 SoftLase units are installed worldwide, according to a notice on the FDA Web site.
Copyright © 2010 DrBicuspid.com