Ormco has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for Insignia, a combination of 3D treatment software and customized orthodontic appliances.
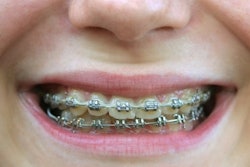
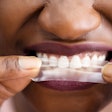
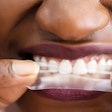
With Insignia, clinicians have the option to choose traditional twins, self-ligating brackets, or aligners that are customized to the individual patient, the company noted. Compatible appliances include Insignia Clearguide Express aligners, Damon Q, Damon Clear, Titanium Orthos, and Inspire ICE.
Insignia uses a high-resolution scan of a PVS impression to create a precise 3D virtual model of the patient's dental anatomy. Utilizing more than 40,000 data points per tooth, unique algorithms in the software define, coordinate, and design the patient's arches into the best possible occlusion. The doctor then uses this initial occlusion to apply a more finite level of design, taking into consideration facial features and treatment preferences.