SomnoMed has received 510(k) marketing clearance from the U.S. Food and Drug Administration for its SomnoDent G2 sleep apnea device.
The company is officially launching the product this week at the Associated Professional Sleep Society meeting in Boston, the company noted in a press release.
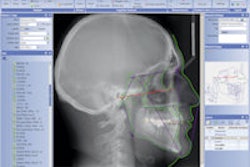
The SomnoDent G2 incorporates modular adjustment parts that are uniquely identified and provide accurate advancement of the G2 splints, according to SomnoMed. The design provides controlled measurement of a patient's mandible position to treat obstructive sleep apnea. The device's range of motion has been extended to provide more treatment options, the company noted.
The SomnoDent G2 is metal-free and 20% smaller than SomnoMed's current range of sleep apnea devices.