OrthoAccel Technologies has received U.S. Food and Drug Administration (FDA) clearance for the AcceleDent tooth movement system and is preparing to launch the product in the U.S.
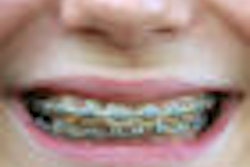
AcceleDent is a removable, noninvasive appliance that a patient with braces wears in the mouth for 20 minutes per day to accelerate orthodontic tooth movement.
A randomized, controlled clinical trial conducted at the University of Texas Health Science Center at San Antonio (UTHCSA) demonstrated that tooth movement could be accelerated by 106% during the initial alignment phase and 38% to 50% during the closure of extraction space. The researchers found no evidence of resorption or any other adverse events caused by the appliance, according to OrthoAccel.
"With an increase in rate of tooth movement observed in our study, the orthodontic treatment time could be shortened by at least five months during the first two stages of orthodontic treatment," stated Dubravko Pavlin, DMD, PhD, an associate professor of orthodontics at UTHCSA and a consultant to OrthoAccel, in a press release.
The first clinical trial on the AcceleDent system was completed under the guidance of Jeryl English, DDS, and Chung Kau, BDS, PhD, at UTHCSA.
OrthoAccel has been selling the AcceleDent system outside the U.S. since October 2009.