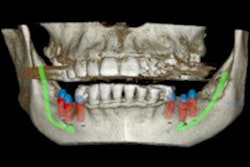
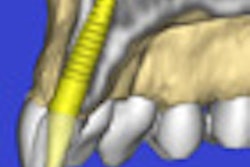
Medical device company X-Nav Technologies has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its X-Guide dynamic 3D navigation system for implants.
The X-Guide system is designed to improve the surgeon's control over the implant process, according to the company. With its treatment software and new, patent-pending X-Point navigation technology, the system provides turn-by-turn guidance during live surgery, using the surgeon's plan. This allows for more exact placement by allowing the visualization of the precise movements of the handpiece during osteotomy and implant delivery, X-Nav said.
"Just as cone-beam [CT] 3D imaging has transformed dental implant procedures for surgeons and patients, this next advancement in technology will bring more precision and control in transferring 3D treatment plans to the patient, with ease," stated Ed Marandola, X-Nav president and CEO, in a press release.
The system is compatible with most cone-beam CT systems, according to the company.
X-Nav has received clearance to market and sell the X-Guide system to dental clinicians in the U.S., Canada, and Europe.