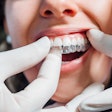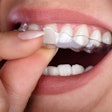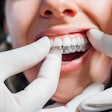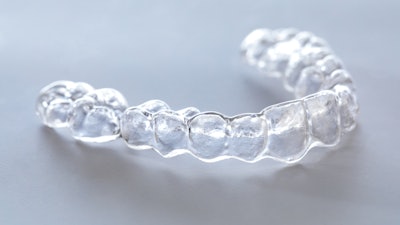
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance to OrthoFX’s NiTime aligners, which are designed for overnight wear.
The new aligners will be available to a limited initial group of orthodontic practices starting on April 21.
OrthoFX will exhibit NiTime aligners at booth No. 641 at the annual session of the American Association of Orthodontists, which will be held in Chicago April 21-24.