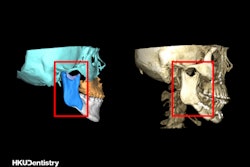
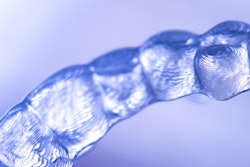
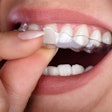
Medical device company Align Technology has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Invisalign treatment with mandibular advancement.
The new clear-aligner system simultaneously aligns teeth and repositions the lower jaw. The treatment replicates the action of functional appliances for class II correction through precision wings that hold the mandible in a forward position while simultaneously correcting malocclusion and crowding issues, according to Align Technology.
The treatment will be available in the U.S. beginning on November 19.