TMJ Health has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its TMJ NextGeneration device for reducing temporomandibular joint disorder (TMJD) pain.
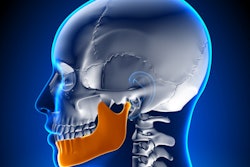
 TMJ NextGeneration device for reducing TMJD pain. Image courtesy of TMJ Health.
TMJ NextGeneration device for reducing TMJD pain. Image courtesy of TMJ Health.The device consists of two custom-made, hollow ear canal inserts that allow the passage of sound and are nearly invisible from the outside, according to the company. The ear canal is located close to the temporomandibular joint (TMJ), and the volume of the ear canal increases when the jaw is opened through movements such as chewing, smiling, and speaking. The TMJ NextGeneration device uses this anatomical change to provide near-field treatment for TMJD.
Patients wearing the devices in a three-month clinical study experienced a significant reduction in the pain and dysfunction associated with TMJD, as much as that experienced by patients wearing a bite splint, the company said (Journal of Craniomandibular Practice, July 2012, Vol. 30:3, pp. 172-181). In addition to the pain reduction, all subjects indicated excellent (71%) or good (29%) overall satisfaction with the device.