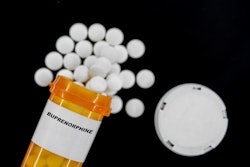
The use of transmucosal buprenorphine, a drug prescribed to treat opioid use disorder and pain, may cause dental problems, according to a warning issued January 12 from the U.S. Food and Drug Administration (FDA).
Reports of dental problems include oral infections, as well as tooth decay and loss, even in patients with no history of oral health issues. The reports have triggered the FDA to require that a new warning about these risks be added to the prescribing information and patient medication guides for medicines that contain buprenorphine and are dissolved in the mouth.
Dentists should evaluate and develop treatment plans for patients taking these medications, according to the FDA.
"Dentists treating someone taking a transmucosal buprenorphine product should perform a baseline dental evaluation and caries risk assessment, establish a dental caries preventive plan, and encourage regular dental checkups," the FDA noted in a drug safety communication.
The use of buprenorphine-containing medicines that are dissolved in the mouth has grown rapidly in recent years. The drugs work by changing the way the body responds to pain and can decrease the pleasurable effects caused by other opioids, which can lead to abuse. The estimated number of buprenorphine prescriptions dispensed from U.S. retail and mail-order pharmacies climbed significantly from 11 million in 2014 to 16 million in 2020, according to the agency.
In 2002, buprenorphine was approved as a drug taken under the tongue to treat opioid use disorder. In 2015, the medication was approved as a film to be placed inside the cheek to treat pain. Other products deliver buprenorphine by other routes, such as via a skin patch or injection, but the FDA has not identified a risk of dental health problems with these forms.
Since buprenorphine was approved, the FDA has identified 305 cases of dental problems -- 131 of which were considered serious -- in patients taking buprenorphine medicines that dissolved in the mouth. However, the number of cases may be underreported. Only those reported to the FDA or published in other literature were included.
In most cases, patients were using the drug to treat opioid use disorder. However, 28 cases of oral health problems occurred in patients taking buprenorphine for pain, and only two of these cases involved people who had a history of dental problems.
In some cases, patients reported dental problems as early as two weeks after the start of treatment. However, the median time to diagnosis was about two years after the start of taking buprenorphine. Healthcare professionals reported most of the cases and provided documentation of extensive dental adverse events, according to the FDA.
In 71 cases, patients underwent tooth extractions, which was the most common treatment reported. In other cases, patients needed root canals, dental surgery, and other treatments such as implants.
In addition to the warning for dental problems, strategies to maintain or improve oral health while taking these medications will be added to prescribing and patient information for the medication, according to the agency.
Dentists should educate patients about potential dental problems, as well as the importance of taking extra steps after the medication dissolves in the mouth. Once the medicine is completely gone, patients should gently rinse their teeth and gums with water and then swallow. Also, patients should wait at least one hour before brushing their teeth to avoid damage, according to the FDA.
To help the FDA track safety issues, healthcare professionals are urged to report side effects involving buprenorphine to the agency's MedWatch program.
"Despite these risks, buprenorphine is an important treatment option for opioid use disorder (OUD) and pain, and the benefits of these medicines clearly outweigh the risks," the agency wrote.