Septodont has acquired the OraVerse product line from Novalar Pharmaceuticals, the companies announced today. OraVerse is the first local anesthesia reversal agent to gain U.S. Food and Drug Administration (FDA) approval.
Under the terms of the agreement, Septodont will take over all sales, marketing, and regulatory responsibilities for all Septodont North American and unpartnered international markets. Septodont will pay Novalar and its investors an upfront payment in addition to milestones and royalties on product sales. Other financial terms of the deal were not disclosed.
Septodont and its North American subsidiary Novocol Pharmaceutical of Canada, with locations in Colorado, Pennsylvania, and Ontario, manufacture and distribute Septocaine and other anesthetics and dental materials.
"OraVerse fits very well within Septodont's portfolio of products and will benefit in particular from its compatibility with their leading proprietary brand Septocaine," said Donna Janson, president and CEO of Novalar, in a press release announcing the acquisition. "Septodont has the established dealer relationships, manufacturing expertise, complementary product lines, and the marketing resources to take OraVerse to the next level."
Novalar received FDA approval for OraVerse in 2008. The FDA considers the drug safe and effective for adults and children 6 years and older and weighing 33 lb or more, according to the company.
In multicenter, double-blinded, randomized, controlled clinical trials of phentolamine mesylate -- the active ingredient in OraVerse -- researchers found that patients who received OraVerse experienced a return to normal sensation and function in about half the time (Journal of the American Dental Association, August 2008, Vol. 139:8, pp. 1080-1093, 1095-1104).
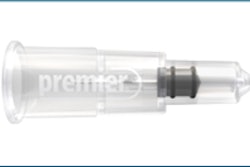
OraVerse comes in a standard dental cartridge and can be injected using the same injection site and an identical technique as that used for local anesthetic. It is used in a 1:1 ratio to local anesthetic and has been tested in doses of one-half, one, and two cartridges.
Last year Novalar partnered with Sanofi-Aventis Deutschland to sell OraVerse in Europe, and with H.A. Systems Dental Imports to distribute OraVerse in Israel.