Editor's note: Second Opinion is a feature where dental leaders and opinion makers have a forum to express their positions on topics relevant to the practice of dentistry. DrBicuspid.com does not take an editorial position on these issues, but we do believe all sides of an issue should have an opportunity to be heard and discussed. We invite you to express your opinion in our Forums or submit a Second Opinion for consideration.
As reported by DrBicuspid.com on March 7, the International Academy of Oral Medicine and Toxicology (IAOMT) filed suit against the U.S. Food and Drug Administration (FDA) last week for its failure to protect the American public from the risks of dental amalgam mercury. Part of the impetus for the IAOMT's legal action was the government's neglect for the well-being of consumers, dental professionals, and the environment, as exhibited by FDA's failure to take timely action against a dental material known to include a potent neurotoxin.
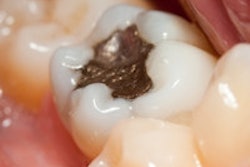
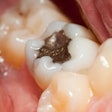
The lawsuit is especially pertinent because patients and dental workers have repeatedly been misled by government authorities and other groups into believing that amalgam fillings, which contain approximately 50% mercury, are not harmful. The IAOMT's claim also establishes that the FDA and the American Dental Association (ADA) have disregarded scientific data about the dangers of using this poisonous substance as a dental restorative material.
“Patients and dental workers have repeatedly been misled by government authorities and other groups into believing that amalgam fillings ... are not harmful.”
In fact, the ADA stated in a 2013 press release about a "Dr. Oz Show" TV segment that "not one credible scientific study supports" the concept that dental amalgam is harmful to health. However, the IAOMT has collected hundreds of studies on the potential hazards of dental mercury amalgam published in peer-reviewed scientific journals. The IAOMT has provided many of these studies to government authorities and dental groups in documented presentations and official correspondences, including expert testimonies at the 2010 FDA Dental Products Panel hearings held to address concerns about dental amalgam.
Although the FDA and ADA have persistently suggested mercury amalgam is not harmful, modern science has confirmed the centuries-old knowledge that mercury is toxic. The most up-to-date research about dental amalgam mercury identifies the harm it can inflict on patients, especially pregnant women, children, fetuses, and individuals with a genetic predisposition to mercury-related reactions. Additionally, dental workers are known to be at high risk as a result of routine occupational exposure to mercury from amalgam placement, maintenance, and removal procedures.
Last year, the IAOMT published a position statement on dental amalgam mercury highlighting the research, and the IAOMT is now in the process of cataloguing all the scientific studies demonstrating the risks of dental amalgam mercury in a publicly accessible database. While this IAOMT Library is a work in progress, due to recent events such as the signing of a United Nations global agreement to phase down industrial mercury, the database is already being shared to raise awareness about amalgam and other dental issues.
In light of this research and the lack of any significant U.S. regulatory action, IAOMT's 2014 lawsuit outlines the failure of the FDA to protect the American people from dental mercury. The need for such regulation essentially traces back to 1976, when Congress passed the Medical Device Amendments Act requiring the FDA to classify all medical devices. It took more than a decade, but finally in 1987, the individual components of amalgam were approved and classified separately with mercury in class I (less risk, no proof of safety needed) and amalgam alloy (the material mixed with the mercury to form amalgam) placed in class II (more risk, no proof of safety needed).
Concerns and deliberations continued until 2002, when the ADA and FDA succeeded in allowing amalgams to be used without a proof of safety, but objections to dental amalgam did not end. Notably, in September of 2006, a joint panel of scientific experts rejected an FDA white paper's assurances of the safety of dental mercury amalgam.
On July 28, 2008, the IAOMT submitted a public comment to FDA demanding dental mercury amalgam be classified in conformance with the mandate of the Medical Device Amendments Act of 1976. Nearly a year later, IAOMT also filed a Citizen's Petition to influence FDA policy-making on amalgam. A few days later, on July 28, 2009, FDA announced (since archived but not altered) it was classifying dental mercury amalgam for the first time in class II without requiring any significant special controls. FDA's Final Rule on this issue was published on August 4, 2009, and an FDA warning for dental mercury amalgam use in pregnant women and developing children was soon removed from FDA's website.
Following the issuance of FDA's Final Rule, the IAOMT sponsored a Petition for Reconsideration in 2009 that identified 29 errors committed by FDA in its discussion of risk assessment principles.
Based on the IAOMT petition, the FDA scheduled a meeting of the Dental Products Panel of the Medical Devices Advisory Committee in December 2010. The panel encouraged the FDA to consider limiting dental mercury amalgam use in pregnant women and children, and to consider labeling that would warn consumers about the risks of this mercury-containing product. Due to promises communicated by the FDA, a decision on the issue was expected from the FDA by December 31, 2011, but no decision has been issued to date.
With particular concern for the more than 67 million Americans who exceed the intake of mercury vapor considered "safe" by the U.S. Environmental Protection Agency (EPA), the lawsuit filed by IAOMT last week charges that the FDA has failed to respond within a reasonable time to petitions calling for either a formal ban of amalgam use or placement in FDA's class III. Such a classification would require additional restrictions for vulnerable individuals, more stringent proof of safety, and an environmental impact statement.
The IAOMT lawsuit holds the FDA accountable for long overdue action to classify dental amalgam accurately to protect American citizens, dental professionals, and the environment as other nations have already done. The scientific evidence of harm from this toxic dental material is clear beyond a reasonable doubt.
Griffin Cole, DDS, is president of the IAOMT; John Kall, DMD, is board chair of the IAOMT; and Amanda Just, MS, is a writer with the IAOMT.
The comments and observations expressed herein do not necessarily reflect the opinions of DrBicuspid.com, nor should they be construed as an endorsement or admonishment of any particular idea, vendor, or organization.