Scientists have fused acoustics and microfluidics in a novel saliva test designed to show within minutes whether a person has a high-risk strain of HPV associated with mouth and throat cancers. Results were published online December 13 in the Journal of Molecular Diagnostics.
Investigators at Duke University, the University of California, Los Angeles (UCLA), and other institutions developed an acoustofluidic noninvasive technique that analyzes saliva for the presence of HPV type 16, the pathogenic strain that causes oropharyngeal cancers (OPCs). Their acoustofluidic platform detected OPC in the saliva of 80% of patients with cancer confirmed by tissue biopsies, reported co-lead investigator Tony Jun Huang, PhD, a professor of mechanical engineering and mechanical science at Duke, and colleagues.
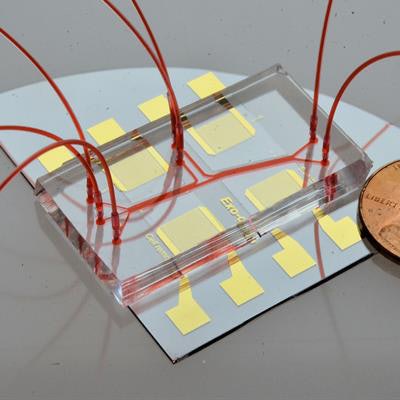
 Acoustofluidic exosome isolation chip for salivary exosome isolation. The microfluidic channel is shown by a red dye solution and a coin demonstrates the size of the chip. Two pairs of gold interdigital transducers are deposited along the channel, which separates particles according to size. Image courtesy of the Journal of Molecular Diagnostics.
Acoustofluidic exosome isolation chip for salivary exosome isolation. The microfluidic channel is shown by a red dye solution and a coin demonstrates the size of the chip. Two pairs of gold interdigital transducers are deposited along the channel, which separates particles according to size. Image courtesy of the Journal of Molecular Diagnostics.OPC rises with HPV
This type of novel rapid-detection method has potential to enable early detection and improve health outcomes. Currently, diagnosis involves clinical examinations -- visual and palpation -- that are unable to pick up premalignant lesions in oral cavities. The incidence of OPC has been rising fast along with HPV, and there are approximately 115,000 new cases reported worldwide every year, the authors noted. The five-year survival rate is less than 50%, underscoring the need for better screening and detection methods.
For the study, the researchers analyzed saliva samples from 10 patients diagnosed with HPV-OPC using traditional methods. They found that their technology combined with Droplet Digital polymerase chain reaction (ddPCR, Bio-Rad) identified HPV16 DNA in 80% of confirmed OPC cases. Reverse transcription PCR analysis showed that the average yield of salivary exosomal small RNA from the acoustofluidic platform is 15 times greater than with the current gold standard: differential centrifugation. Essentially, the acoustofluidic platform can achieve high-purity, high-yield salivary exosome isolation for downstream salivary exosome-based liquid biopsy applications.
And because isolation is derived using low power intensity surface acoustic waves, it's gentler than long-term exposure to a high centrifugal force. So, the platform can isolate structurally intact and biologically active exosomes.
Moreover, the genomic and proteomic profiling efficiency of liquid biopsy is significantly optimized by the high purity and yield properties of acoustofluidics, which make it possible to examine rare exosomal microRNAs and protein signatures in the saliva of patients who have OPC.
Isolating salivary exosomes
Exosomes are tiny microvesicles originating within cells secreted into body fluids. Their numbers are elevated with the onset of different types of cancers. In acoustofluidics, fluid samples are analyzed by a small acoustofluidic chip that isolates salivary exosomes by removing unwanted particles based on size, leaving exosome-rich concentrated samples that make it easier to detect tumor-specific biomarkers.
The researchers optimized their acoustofluidic platform for isolating exosomes in saliva samples that had different physical qualities, including different viscosities. Because the scientists' high-yield exosome isolation approach can tolerate variability among samples caused by changes in saliva viscosity and collection methods used, it may be useful in clinical settings.
"These data showed that this platform is capable of consistently isolating exosomes from saliva samples, regardless of viscosity variation and collection method," Huang and colleagues wrote.
Speeding detection
The scientists developed their diagnostic based on the assumption that salivary exosomes are packaged with HPV-associated biomarkers, and that efficiently enriching the exosomes by isolating them could enhance diagnostic and prognostic performance of a test for HPV-OPC. The new automated and fast isolation technique requires less than five minutes of processing time, compared with approximately eight hours of processing time using benchmark technologies.
Acoustofluidics automates exosome isolation and eliminates multistep protocols that would require several instruments and trained technicians. In addition, the 10 to 20 minutes needed for turnaround and the small amount of sample required enhance biosafety and would make high-throughput screening possible in extensive patient populations, the authors noted.