Vigilant Biosciences is debuting its OncAlert oral cancer risk assessment system at this week's International Dental Show (IDS) in Cologne, Germany.
The system includes the OncAlert rapid, point-of-care (POC) risk assessment test, OncAlert POC Test, and the OncAlert oral cancer CD44 + total protein lab test, OncAlert Lab Assay. Both products are based on patented technology that detects specific protein markers known to indicate an elevated risk for oral cancer, even before the observation of visual or physical symptoms. The simple, oral rinse procedure is easy to administer and noninvasive for patients, according to the company.
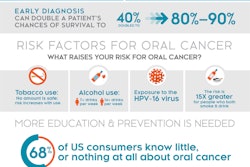
The test can benefit everyone, with particular emphasis on high-risk populations (i.e., current and former tobacco users, those who consume alcohol, and people with human papillomavirus [HPV]). Both the POC test and the lab assay are currently under the European CE Mark registration approval process and expected for release in the second quarter of 2015.
The company also announced that its chief dental officer, John Comisi, DDS, will present an oral presentation, "Improving Early Detection of Oral Cancer with Salivary Diagnostics," on March 12 at the IDS.
In other company news, Vigilant has entered into a three-year exclusive sales and marketing agreement with Biotech-IgG to distribute the OncAlert system in Denmark, Norway, and Sweden.
Headquartered in Lund, Sweden, Biotech-IgG supplies products for medical diagnostics and research, routine, and process applications for the life science industry. Biotech-IgG will market and sell the OncAlert POC test and OncAlert lab assay to the dental and oncology markets in Scandinavia, pursuant to the CE Mark registration approvals.
The company has also entered into a five-year exclusive sales and marketing distribution agreement with BL&H for sale of its OncAlert system in Korea. Located in Seoul, BL&H is a distributor of pharmaceuticals and hospital-based products and services in the Korean market.
Under the terms of the agreement, BL&H will exclusively market and sell the OncAlert POC Test and OncAlert LAB Assay to the Korean dental, otorhinolaryngology, and oncology markets, pending approval of the OncAlert system by the Korean Ministry of Food and Drug Safety. The distribution deal represents more than $5 million in guaranteed purchase commitments, as well as milestone payments to Vigilant, according to the company. The OncAlert system is expected to be available for sale in Korea by the second half of 2015 or early 2016.