Gene therapy can be performed safely in the human salivary gland, which could lead to new treatments for cancer patients suffering from xerostomia, according to scientists at the National Institute of Dental and Craniofacial Research (Proceedings of the National Academy of Sciences, November 5, 2012).
This finding, which comes from the first phase I clinical study of gene therapy in a human salivary gland, has great potential to help head and neck cancer survivors who battle with chronic dry mouth, according to the researchers.
Aquaporin-1 encodes a protein that naturally forms pore-like water channels in the membranes of cells to help move fluid, such as occurs when salivary gland cells secrete saliva into the mouth.
The initial results clear the way for additional gene therapy studies in the salivary glands. Although sometimes overlooked, salivary glands present an ideal target for gene therapy. They are easily accessible, and once a gene is introduced, it has no obvious escape route into the bloodstream, where it can have unintended consequences.
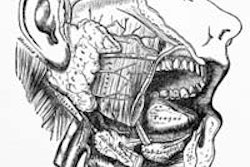
The scientists gave 11 head and neck cancer survivors a single-dose injection of the
aquaporin-1 gene directly into one of their two parotid salivary glands. The gene was packaged in a disabled, nonreplicating adenovirus.
The scientists found that five participants had increased levels of saliva secretion, as well as a renewed sense of moisture and lubrication in their mouths, within the study's first 42 days, the period covered in this report. Of the six who didn't benefit from gene therapy, none had serious side effects. The most common side effect was a transient and relatively minor immune response against the disabled adenovirus.