A "rinse and spit" test for early detection of oral cancer performed well in a clinical feasibility study, according to Vigilant Biosciences, the company commercializing the product.
The study, sponsored by the National Cancer Institute, included 300 subjects in a case-control design that ensured cases (oral cancer patients) and controls (volunteers without cancer) were similar with respect to other factors, such as tobacco and alcohol use, age, and race. It was the largest oral cancer marker study of its kind in the U.S., according to Vigilant.
The principal investigator of the study is Elizabeth Franzmann, MD, an associate professor of otolaryngology at the University of Miami Miller School of Medicine, where the patented technology was developed.
The goal of the study was to determine whether the markers could effectively distinguish individuals with oral cancer from those without cancer.
"Based on preliminary results, we have met our end points and look forward to publishing the results," Dr. Franzmann stated in a press release.
Based on the early study results, which will be submitted to a peer-reviewed medical journal for publication, the company is now pursuing product development and seeking investment capital.
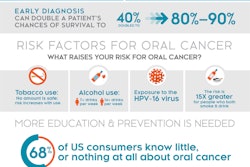
The product consists of a low-cost, oral cancer-specific oral rinse test strip that provides an immediate and simple color change in the presence of certain levels of proteins that have clinically proved to be associated with the early onset of oral cancer.
The test targets smokers, drinkers, and people with human papillomavirus (HPV). It works by measuring early cancer markers via a saline solution that is swished in the mouth and collected in a tube. Collecting the teaspoon-sized sample takes about 10 seconds, and results are designed to be available in minutes, the company noted.