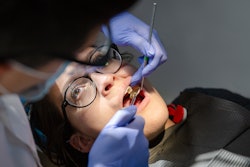
Sugar-coated gold nanoparticles may wipe out stubborn bacteria that get lodged in teeth and potentially eliminate biofilms quicker than antibiotics, according to the U.S. National Institute of Biomedical Imaging and Bioengineering.
The animal study highlights the diagnostic and treatment potential of these newly developed nanoparticles on teeth and the possibilities they hold in reducing antibiotic resistance, according to the agency press release dated March 12.
"The treatment method is especially fast for the oral infection,” Maryam Hajfathalian, PhD, a professor of biomedical engineering at the New Jersey Institute of Technology, said in the release. "We applied the laser for one minute, but really in about 30 seconds we're killing basically all of the bacteria."
Tooth decay-causing bacteria like Streptococcus mutans (S. mutans) create a dense network of proteins and carbohydrates that block antibiotics from reaching the microbes. However, gold nanoparticles can jump this hurdle due to their ability to convert light energy into heat, which is ideal for killing pathogens via photothermal therapy, according to the release.
To further investigate this effect, researchers enclosed gold spheres within larger cage-shaped nanoparticles made of gold to enhance their reaction to light, which allowed biofilms to be imaged and treated.
To attract bacteria, particles were coated with dextran, a carbohydrate commonly found in biofilms, and they were applied to rats' teeth that were infected with S. mutans.
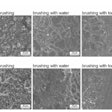
 In the study, gold nanoparticles delivered to infected teeth and skin wounds are heated with near infrared lasers to destroy biofilms. Images and captions courtesy of Hajfathalian et al. Licensed under CC BY 4.0.
In the study, gold nanoparticles delivered to infected teeth and skin wounds are heated with near infrared lasers to destroy biofilms. Images and captions courtesy of Hajfathalian et al. Licensed under CC BY 4.0.
During a photoacoustic imaging test on the teeth, the nanoparticles produced clear signals, enabling the researchers to pinpoint where the biofilms had absorbed the dextran-coated particles on the teeth. As a comparison, they treated other infected teeth samples with the topical antiseptic chlorhexidine, according to the release.
Photothermal therapy was almost completely successful in eliminating biofilms, whereas chlorhexidine had minimal impact on reducing bacterial viability, they found.
 Researchers were able to visualize biofilms in teeth treated with dextran-coated gold nanoparticles using photoacoustic imaging.
Researchers were able to visualize biofilms in teeth treated with dextran-coated gold nanoparticles using photoacoustic imaging.
However, the study had limitations. Further tests are needed to determine whether the approach can prevent cavities or accelerate the healing process, according to the release.
"I think it's important to see how inexpensive, straightforward, and fast this process is," Hajfathalian added. "Since we are limited in using antibiotics, we need novel treatments like this as a replacement."