In a recent study of patients with chronic periodontitis, oral bacteria significantly differed in patients who also had diabetic nephropathy, a common diabetes-related kidney complication. One oral bacteria species in particular may be able to signal patients with renal involvement.
Researchers looked at the saliva samples of 30 patients with diabetes and chronic periodontitis. They used a database to analyze and compare oral microbiomes between 15 patients with diabetic nephropathy and 15 patients without renal disease.
The study is believed to be the first to provide evidence of the potential role of oral bacteria in the development of kidney complications in patients with type 2 diabetes, the authors noted.
"Our findings may help identify whether changes in some specific bacteria or salivary microbiome are associated with the development of [diabetic nephropathy] and periodontitis," wrote the authors of the report, led by Dongxue Zhang from Beijing Chao-Yang Hospital in China (BMC Oral Health, January 16, 2022, Vol. 22, e12).
Patients were recruited from an outpatient section of the department of medicine at Pinggu Hospital in Beijing from July to August 2017. To be eligible, patients had to be diagnosed with type 2 diabetes mellitus, be 30 to 75 years of age, have at least five natural teeth, and sign a written consent form.
The authors excluded patients who were pregnant or lactating, had type 1 diabetes, had a history of periodontal treatment in the last six months, or were using anti-inflammatory drugs or antibiotics in the last three months.
Zhang and colleagues enrolled 169 patients in the study, then matched a final group of 30 patients based on age, sex, body mass index, periodontal status, and blood sugar metrics. Half of the cohort had diabetic nephropathy, and each group of 15 patients had five patients with mild periodontitis, five with moderate periodontitis, and five with severe periodontitis.
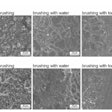
The authors collected saliva samples from the patients, which were analyzed and referenced against a database to determine the type of oral bacteria and compare differences between the two groups. The groups' oral microbiomes differed significantly with respect to the Gemella, Selenomonas spp, Lactobacillales unclassified, Bacteria unclassified, and Abiotrophia species.
The abundance of S. spp. was higher in the group with diabetic nephropathy, whereas Gemella, L. unclassified, B. unclassified, and Abiotrophia were more prevalent in the group without nephropathy. Also, the group with nephropathy showed lower diversity of oral bacteria.
Additional analysis showed that S. spp. may be a potential biomarker to signal diabetic nephropathy in patients with type 2 diabetes. This type of G-negative, anaerobic bacteria has been previously linked to peri-implant health.
"Increased relative abundance of Selenomonas spp. may be associated with developing renal complications of [type 2 diabetes with chronic periodontitis]; this oral microbiome characteristic may serve as a potential marker of [diabetic nephropathy] in patients with [chronic periodontitis]," wrote the authors of the report.
The link between S. spp. and diabetic nephropathology isn't entirely clear. However, the group with renal disease had slightly higher levels of urea nitrogen, which may suggest an imbalance of bacteria in the intestinal tract, the authors noted.
The study had limitations, including its small sample size. It also did not look at the impact of changes in oral flora after periodontal treatment, and patients without diabetes or periodontitis were not included in the research.
"Characterization of the differences of oral microflora ... can provide novel insights about the early treatment and prevention of [diabetic nephropathy]," the authors concluded. "Future studies should include a larger sample size and perform metagenome sequencing to provide more comprehensive results."