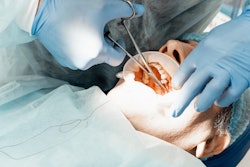
Flightpath Biosciences and the Forsyth Institute will co-develop new products for the prevention and treatment of oral disease, specifically gum disease, based on the life science firm’s antibiotic candidate.
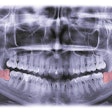
Flightpath’s orally bioavailable, narrow-spectrum antibiotic FP-100 has shown potential to eradicate the causal pathogens of periodontal disease, Treponemes and Fusobacterium. The companies said the antibiotic has been shown to eliminate these pathogens, which are associated with other systemic diseases, without disrupting the gut bacteria and with no evidence of biologically relevant toxicity in multiple animal species.
A phase I clinical trial of FP-100 is expected to start in the first quarter of 2024.