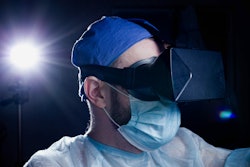
The U.S. Food and Drug Administration (FDA) has granted 510(k) approval to NuLoupes, augmented reality dental and medical loupes from NuEyes.
Using NuEyes' proprietary patent-pending camera system, NuLoupes aims to provide live 3D stereoscopic imaging with near-zero latency. The camera system will deliver submillimeter accurate depth perception to provide dentists and doctors with a natural view and enhanced magnification. The loupes’ high-resolution variable digital magnification aims to provide clinicians with more versatility and a detailed viewing area, according to the company.
For a short time, NuEyes is offering a limited number of NuLoupes developer kits for software developers, clinicians developing medical applications, or anyone who wants access before the loupes come to market. The kit will come with access to the NuLoupes 3D stereoscopic camera sensors, an infrared sensor, and an inertial measurement unit. An Android operating system, unity plug-ins, and more and will be announced soon. The developer kits are projected to ship in the first quarter of 2024.