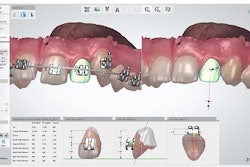
3Shape has received U.S. Food and Drug Administration (FDA) 510(k) market clearance for additional aspects of its 3Shape Ortho System software.
The clearance applies to the design of dental retainers, splints, mouthguards, and nightguards, according to the company.
This follows on the company's recent announcements that its indirect bonding application will now digitally integrate with Rocky Mountain Orthodontics' and American Orthodontics' bracket systems.